A kind of s-nitrosothiol/polysaccharide-based in-situ forming hydrogel and its preparation method and application
A nitrosothiol and in-situ molding technology, which is applied in pharmaceutical formulations, prostheses, bandages, etc., can solve the problems of low NO load, ineffective antibacterial effect, and difficult control of controlled release, achieving rapid response, Abundant sources, the effect of promoting wound healing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1: the preparation of thiol sodium alginate
[0055] Dissolve 1 g of sodium alginate (SA) powder in 100 ml of deionized water, add morpholinoethanesulfonic acid (MES) to make the concentration 0.1 mol / L, and adjust the pH to 6.0 with 1 mol / L HCl solution. According to the final feeding ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDAC): N-hydroxysuccinimide (NHS): COO - =1:1:1 molar ratio, add EDAC and NHS in sequence, and use hydrochloric acid (HCl) solution (1mol / L) to adjust the system pH=5.0~6.0. Under dark conditions, stir at room temperature for 20 minutes, and then follow the n-NH 2 / n-COOH=1:1 molar ratio, add cysteine hydrochloride to the reaction system, and use sodium hydroxide (NaOH) solution (1mol / L) to adjust the system pH=5.0. Also under dark conditions, stirred at room temperature for 10h, the reaction solution was placed in a dialysis bag (φ30, 5000 ~ 8000KDa), respectively with HCl solution (pH = 5.0), containing 1% (wt.%)...
Embodiment 2
[0056] Embodiment 2: Preparation of mercaptolated hyaluronic acid
[0057] Dissolve 0.5g of hyaluronic acid (HA) powder in 100ml of deionized water, according to the final dosage of ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDAC): N-hydroxysuccinate Imide (NHS): COO - =1:1:1 molar ratio, add EDAC and NHS in sequence, and use hydrochloric acid (HCl) solution (1mol / L) to adjust the system pH=5.0~6.0. Under dark conditions, stir at room temperature for 20 minutes, and then follow the n-NH 2 / n-COOH=1:1 molar ratio, add cysteine hydrochloride to the reaction system, and use sodium hydroxide (NaOH) solution (1mol / L) to adjust the system pH=5.0. Also under dark conditions, stirred at room temperature for 10h, the reaction solution was placed in a dialysis bag (φ30, 5000 ~ 8000KDa), respectively with HCl solution (pH = 5.0), containing 1% (wt.%) sodium chloride ( NaCl) HCl solution (pH=5.0) and HCl solution (pH=5.0) were dialyzed at 4°C in the dark for 1 day, f...
Embodiment 3
[0060] Embodiment 3: the preparation of S-nitrosothiol
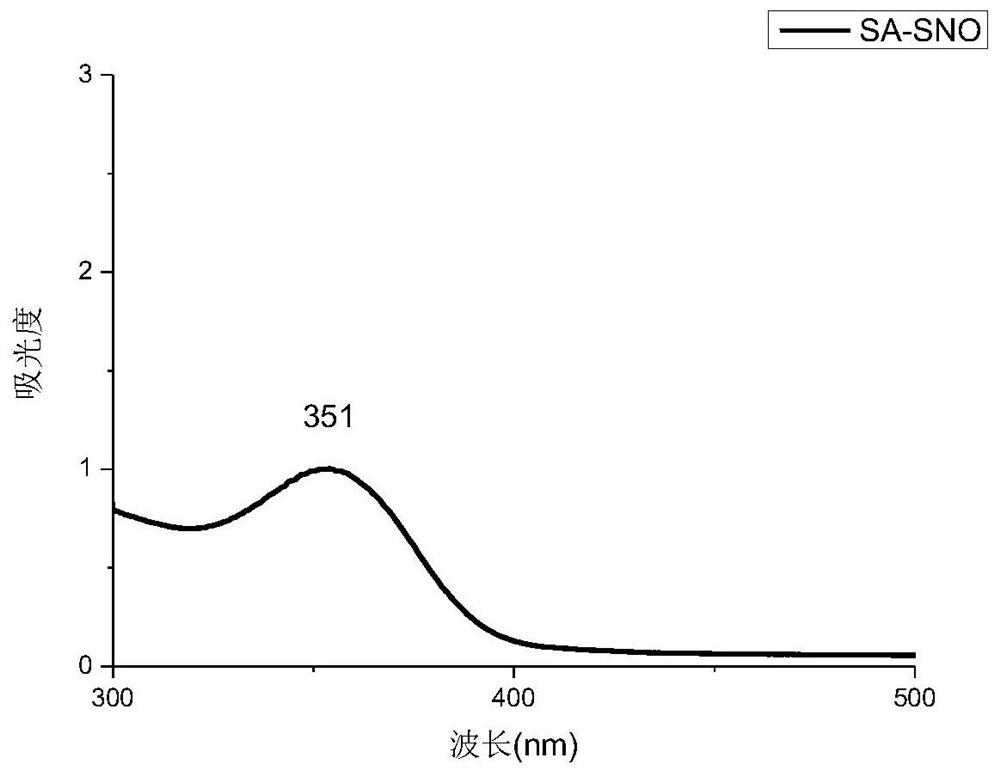
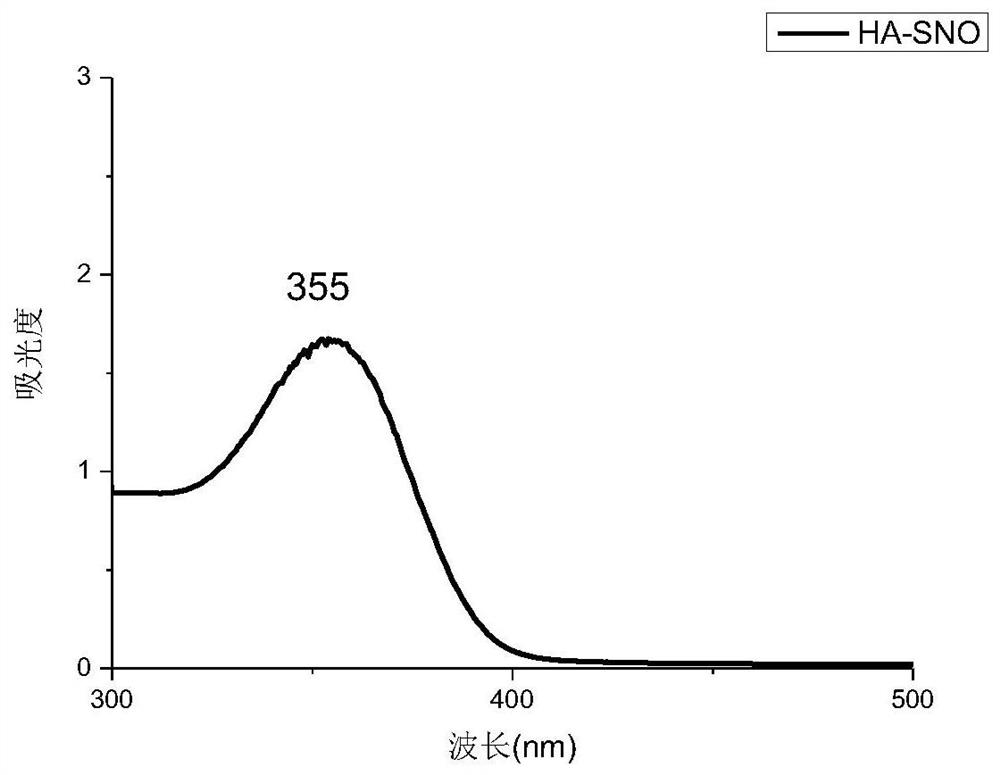
[0061] Two 100ml aqueous solutions of thiolated polysaccharides (SA-SH and HA-SH aqueous solutions, both of which have a concentration of 0.005g / ml) were adjusted to pH 3.5 with 1mol / L HCl, and cooled to about 0°C in an ice bath . Protect it from light with aluminum foil. Then add 0.5g NaNO respectively 2 React with thiolated polysaccharides at 0°C for 1.5 h in the dark. The reaction solution (S-nitrosothiol) was dialyzed against deionized water for 10 hours using a 3500 MWCO membrane in an ice bath under dark conditions. After purification, the R-SNO solution was lyophilized and stored at −20 °C to obtain SA-SNO and HA-SNO, respectively. Such as figure 1 , 2 As shown, there is a characteristic peak at around 350nm in the ultraviolet spectrum, and the peak at 350nm indicates the n 0→p* electronic transition of the S–NO bond, which proves that the product has an S-NO bond. From the above table 1, the sulfhydryl con...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com