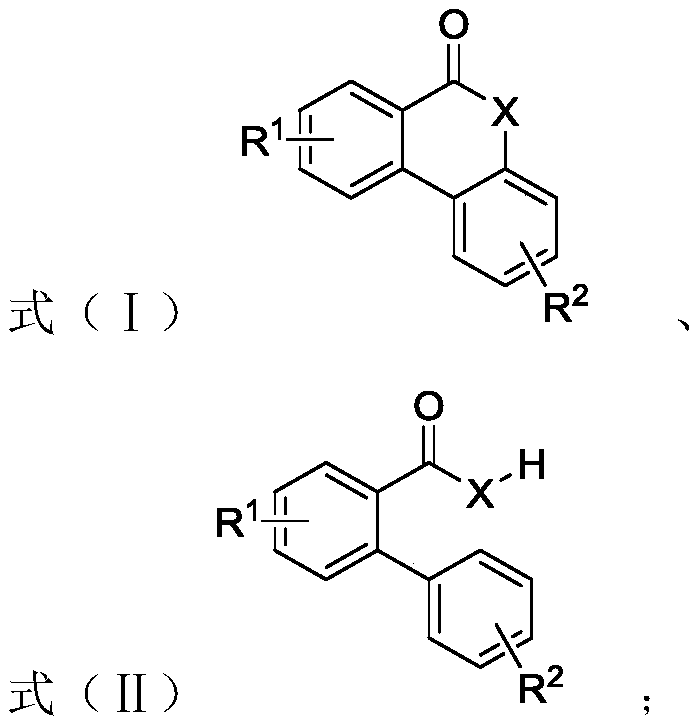
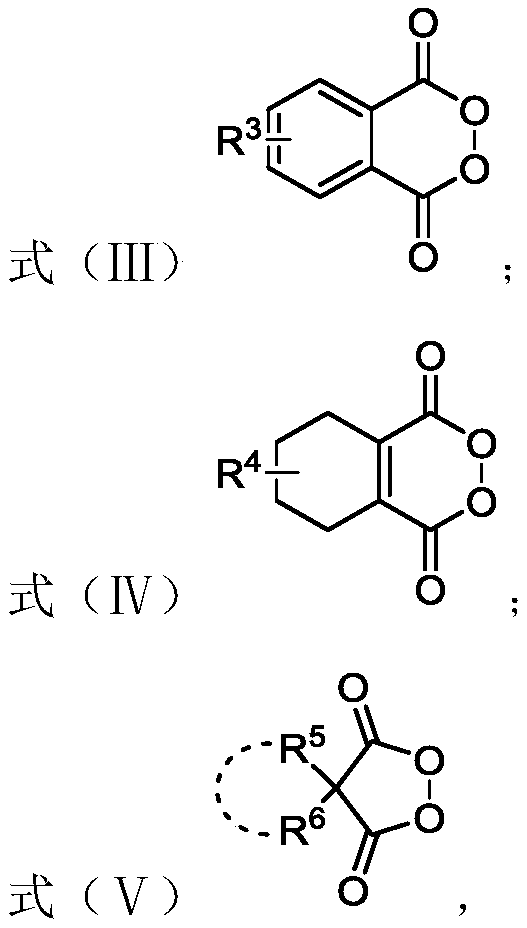
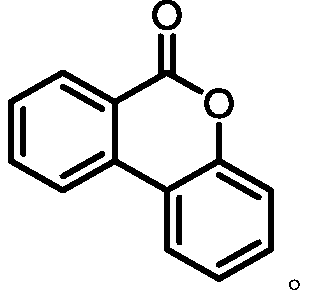
Preparation method of six-membered aryl lactone or six-membered aryl lactam compound
A technology of aryl lactam and aryl lactone, applied in the direction of organic chemistry and the like, can solve the problems of lack of synthesis method, lack of preparation method of six-membered aryl lactone or six-membered aryl lactam compound, unfriendly environment, etc. , to achieve the effect of high atom utilization, high yield and suitable solvent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Add 0.36mmol cesium iodide (CsI) and 0.3mmol 2-phenylbenzoic acid into a 25mL reaction tube, add 0.6mmol MPO (4,4-dimethyl-1,2-dioxolane-3,5-dione) and 3mL of o-dichloroethane, the molar ratio of cesium iodide to MPO is 0.6:1; use a vacuum pump to remove the gas in the reaction tube and solvent, and fill it with nitrogen, repeat the operation three times, and then place the reaction tube at a temperature of 420-430nm In the stirrer irradiated by the blue light of the band LED, the reaction is detected with a thin-layer chromatography (TLC) plate until the reaction of the raw materials is complete;
[0033] (2) Add 10mL of ethyl acetate (EA) and 10mL of water, extract the liquid with a 60mL separating funnel, extract the aqueous phase with EA three times (3*10mL), combine the organic phase and wash once with 10mL of saturated brine, collect The organic phase was dried with anhydrous sodium sulfate;
[0034] (3) Remove sodium sulfate by filtration, and the organic ph...
Embodiment 2
[0042](1) Add 0.36mmol cesium iodide (CsI) and 0.3mmol N-methoxy-1,1'-biphenyl-2-amide into a 25 mL reaction tube, add 0.6mmol MPO (4,4-dimethyl- 1,2-dioxolane-3,5-dione) and 3mL o-dichloroethane, use a vacuum pump to remove the gas in the reaction tube and solvent, and fill it with nitrogen, repeat the operation three times, and then place the reaction tube in the 420-430nm band In the stirrer irradiated by LED blue light, use a thin layer chromatography (TLC) plate to detect the reaction until the reaction of the raw materials is complete;
[0043] (2) Add 10mL of ethyl acetate (EA) and 10mL of water, extract the liquid with a 60mL separating funnel, extract the aqueous phase with EA three times (3*10mL), combine the organic phase and wash once with 10mL of saturated brine, collect The organic phase was dried with anhydrous sodium sulfate;
[0044] (3) Sodium sulfate was removed by filtration, and the organic phase was removed by a rotary evaporator to obtain a crude produc...
Embodiment 3
[0052] This example only describes the difference from Example 1, the solvent in the step (1) is different, specifically dichloromethane, and the yield of the crude product is 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com