Method for oxidizing yellow phosphorus to remove arsenic
A yellow phosphorus and arsenic removal technology, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc. Issues such as unsatisfactory production efficiency and safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation of oxidant solution: 10wt% H 2 o 2 , 0.1wt% phosphoric acid and 89.9wt% water were uniformly mixed to obtain an oxidizing agent solution;
[0038] Prepare oxidation catalyst solution: mix 1wt% KI and 99wt% water evenly to obtain oxidant catalyst solution;
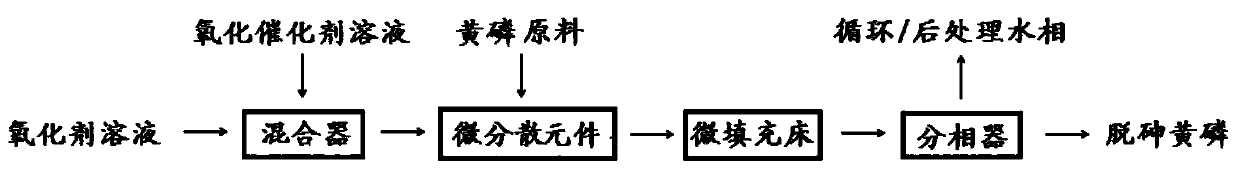
[0039] The oxidant solution and the oxidation catalyst solution were pre-mixed continuously for 1 s at a flow ratio of 8:1 using a micro-mixer to obtain a mixed fluid; Mix in the element, redox reaction in the micro-packed bed at 70°C for 5 minutes, place the obtained reaction system in a phase separator for phase separation, and obtain the aqueous phase and the crude yellow phosphorus (lower phase), and the crude yellow phosphorus Wash with 3wt% hydrogen peroxide to obtain dearsenic yellow phosphorus; supplement H in the obtained water phase 2 o 2 , phosphoric acid and KI to its initial concentration, to obtain the aqueous phase circulation liquid, and recycle;
[0040] The arsenic content in the aq...
Embodiment 2
[0042] Preparation of oxidant solution: 30wt% H 2 o 2 , 0.1wt% phosphoric acid and 69.9wt% water were uniformly mixed to obtain an oxidizing agent solution;
[0043] Prepare oxidation catalyst solution: mix 2wt% KI and 98wt% water evenly to obtain oxidant catalyst solution;
[0044] The oxidant solution and the oxidation catalyst solution were premixed continuously for 0.1s at a flow ratio of 4:1 by a micro-mixer to obtain a mixed fluid; Mix in a micro-dispersion element, redox reaction in a micro-packed bed at 90°C for 1 min, place the resulting reaction system in a phase separator for phase separation, and obtain an aqueous phase and crude yellow phosphorus (lower phase). The crude phosphorus product is washed with 6wt% hydrogen peroxide to obtain dearsenic yellow phosphorus; the obtained aqueous phase is supplemented with H 2 o 2 , phosphoric acid and KI to its initial concentration, to obtain the aqueous phase circulation liquid, and recycle;
[0045] The arsenic cont...
Embodiment 3
[0047] Preparation of oxidant solution: 30wt% H 2 o 2 , 0.03wt% phosphoric acid and 69.97wt% water were uniformly mixed to obtain an oxidizing agent solution;
[0048] Prepare oxidation catalyst solution: mix 0.5wt% KI and 99.5wt% water uniformly to obtain oxidant catalyst solution;
[0049] The oxidant solution and the oxidation catalyst solution were premixed continuously for 0.5s at a flow ratio of 5:1 by a micro-mixer to obtain a mixed fluid; Mix in the dispersing element, redox reaction in the micro-packed bed at 60°C for 1 min, put the obtained reaction system in a phase separator for phase separation, and obtain the aqueous phase and the crude yellow phosphorus (lower phase), and the yellow phosphorus The crude product is filtered with a 3 μm sand core funnel to obtain the dersensic yellow phosphorus; the obtained aqueous phase is supplemented with H 2 o 2 , phosphoric acid and KI to its initial concentration, to obtain the aqueous phase circulation liquid, and recy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com