A kind of fluoroquinolone antibiotic capsule for pet and preparation method thereof
A fluoroquinolone and antibiotic technology, which is applied in the field of pet fluoroquinolone antibiotic capsules and its preparation, can solve the problems of inability to achieve enteric coating effect, and achieve convenient clinical administration, good stability, and improved bioavailability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
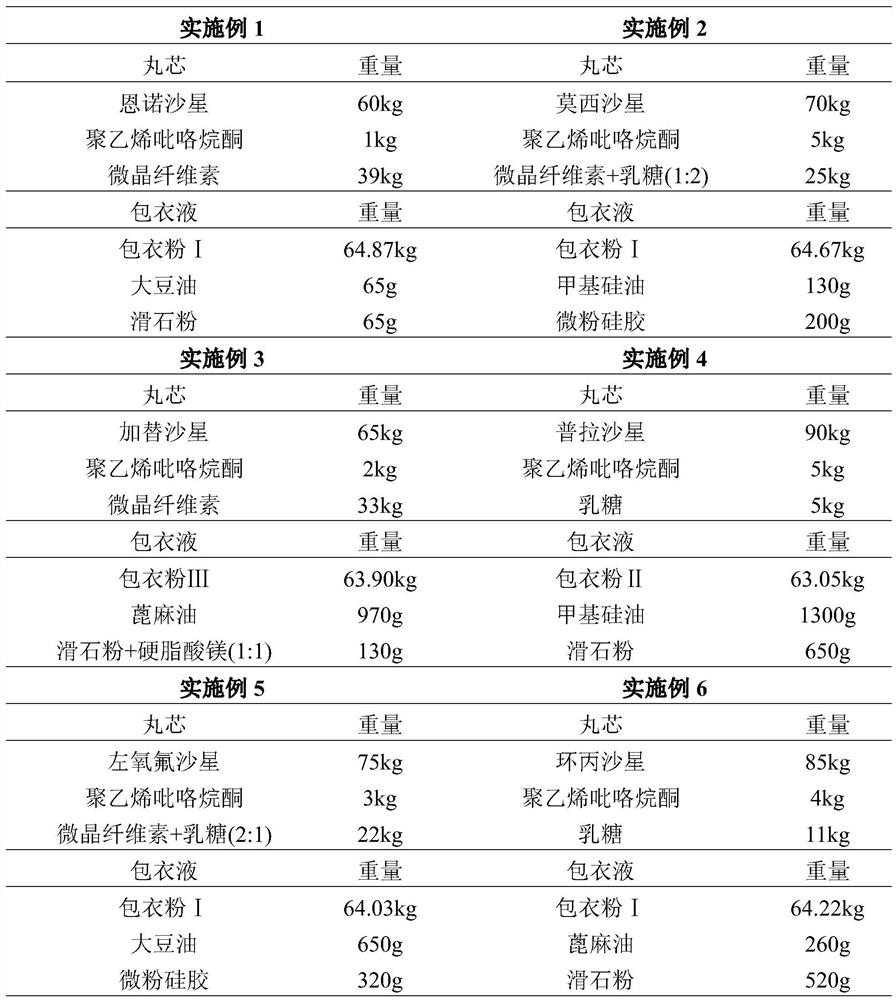
Embodiment 1-6
[0031] Embodiment 1-6 Fluoroquinolone antibiotic capsule specific prescription and dosage
[0032]
Embodiment 1~6
[0033] The preparation method of embodiment 1~6 is as follows:
[0034] (1) Handling and weighing of raw and auxiliary materials: Pass the raw materials and all auxiliary materials through an 80-mesh sieve respectively for use; weigh all raw materials and auxiliary materials according to the prescribed amount and mark them well.
[0035] (2) Preparation of coating liquid: soak the coating powder in 95% ethanol for 24 hours overnight, add stabilizer and stir for at least 10 hours, add lubricant and keep stirring before use.
[0036] (3) Granulation and drying: the fluoroquinolone raw materials and the filler are double-diluted, and after passing through an 80-mesh sieve three times, a 20% aqueous binder solution is added for mixing, and the granulation and spheronization are carried out with an extrusion spheronizer. and dried at 50°C for 2 hours.
[0037] (4) Fluidized bed coating: the atomization pressure is 1.5 to 3.0 bar, the spray rate is 5 to 15 g / min, the inlet air temp...
Embodiment 7
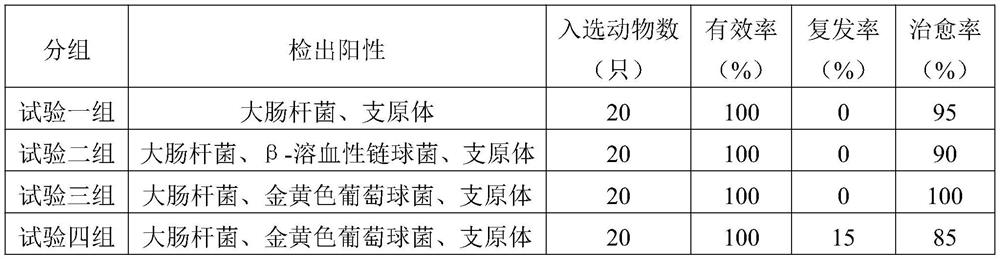
[0042] Example 7 In vitro simulated intestinal release test
[0043] Determination method of release rate: According to the second method of release rate determination method in the appendix of "Veterinary Pharmacopoeia of the People's Republic of China" 2015 edition, high performance liquid chromatography was used for sample injection measurement, and the release rate of the capsule preparation prepared in the Example was determined.
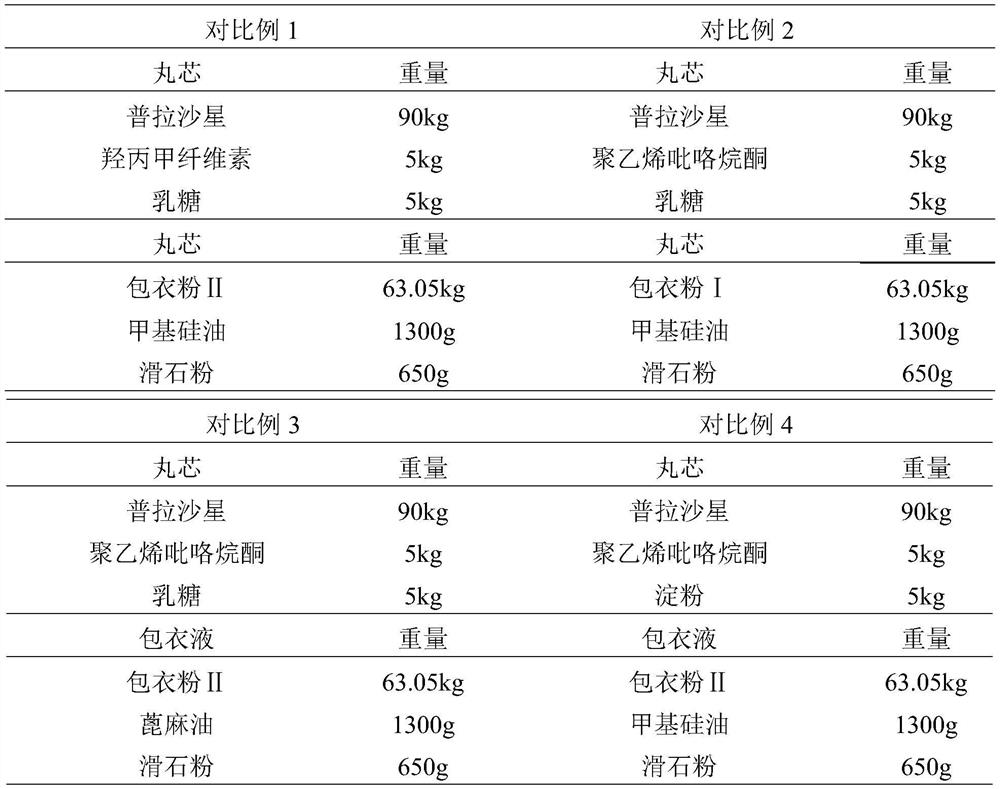
[0044] Test material: prepare the enteric-coated capsule preparation of prafloxacin according to the method in Example 4, commercially available ciprofloxacin capsules for human use in 0.25g specifications, Comparative Example 1, Comparative Example 2 (blank capsules are used for each sample) shell for dispensing to meet the testing amount required by the Veterinary Pharmacopoeia).
[0045] test results:
[0046] Dissolution testing of capsule preparations
[0047] preparation Dissolution % in acid Dissolution % in Alkali Ex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com