Injection dosage form, preparation method and purpose of clopidogrel and salt thereof
A technology of clopidogrel and injection, applied in the direction of non-active ingredient medical preparations, medical preparations containing active ingredients, pharmaceutical formulations, etc., can solve the irreversible anti-platelet aggregation effect, insufficient threshold of active metabolite formation, tablet Slow onset and other issues, to achieve the effect of rapid anti-platelet aggregation, rapid anti-platelet, and high clinical safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
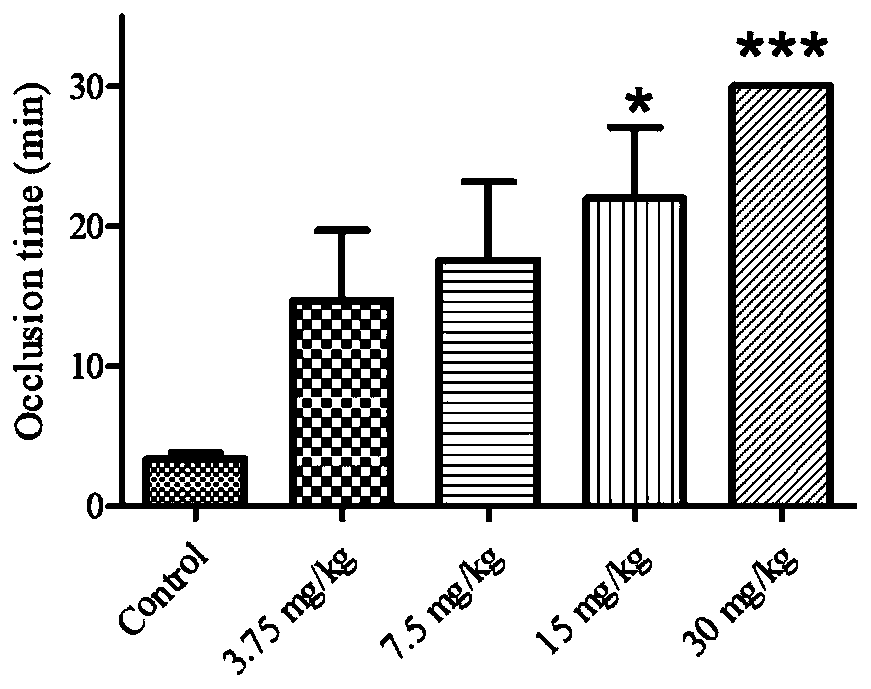
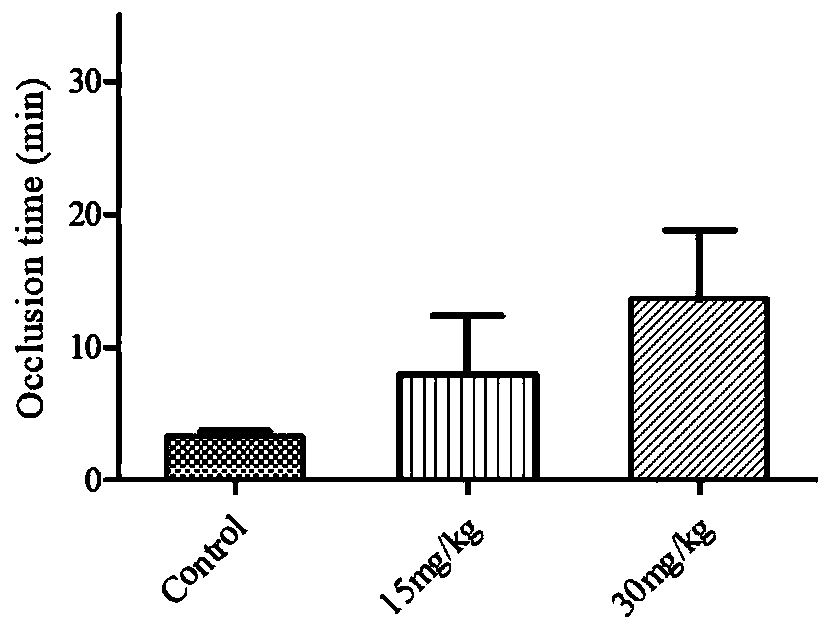
Image
Examples
Embodiment 1
[0028] (1) Synthesis and characterization of mPEG-PDLLA
[0029] Take 10g of mPEG 2000 and lactide each, place them in a 250mL two-neck bottle, and slowly raise the temperature to 135°C, and cool down to 110°C after mPEG2000 and lactide are completely melted. Add 20% (v / v) toluene solution of stannous octoate (the addition of stannous octoate is 0.25% of the total weight of the reactant) as a catalyst, after magnetic stirring and mixing, vacuum remove toluene and residual moisture until the reaction solution has no bubbles generated, heated to 140°C, and reacted for 6h under the protection of nitrogen. After the reaction was completed, after cooling to room temperature, an appropriate amount of dichloromethane was added to the reactant to dissolve, precipitated with low-temperature anhydrous ether, and filtered under reduced pressure to obtain a white filter cake. The purification was repeated 3 times, and the obtained product was vacuum-dried at room temperature for 24 hours...
Embodiment 2
[0033] Synthesis of mPEG-PDLLA micellar nanosystems encapsulating clopidogrel and its salts: Precisely weigh 20 mg of clopidogrel and its salts and 140 mg of mPEG-PDLLA in a vial, ultrasonically dissolve them with an appropriate amount of methanol, and transfer them to an eggplant-shaped bottle , 40°C rotary evaporation until the solvent evaporates and the film is completely formed, then add 2 mL of up water to hydrate, and pass through a 0.22 μm filter membrane to obtain the micellar injection of clopidogrel and its salt.
[0034] The experimental steps and conditions for the synthesis and characterization of mPEG-PDLLA are the same as in Example 1.
Embodiment 3
[0036] Synthesis of mPEG-PDLLA micellar nanosystems loaded with clopidogrel and its salts: Precisely weigh 20 mg of clopidogrel and 200 mg of mPEG-PDLLA into a vial, dissolve them in an appropriate amount of methanol ultrasonically, transfer them to an eggplant-shaped bottle, and keep at 40°C Rotary evaporate until the solvent is evaporated and the film is completely formed, then add 4 mL of up water for hydration, and pass through a 0.22 μm filter membrane to obtain a micellar injection of clopidogrel and its salt.
[0037] The experimental steps and conditions for the synthesis and characterization of mPEG-PDLLA are the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com