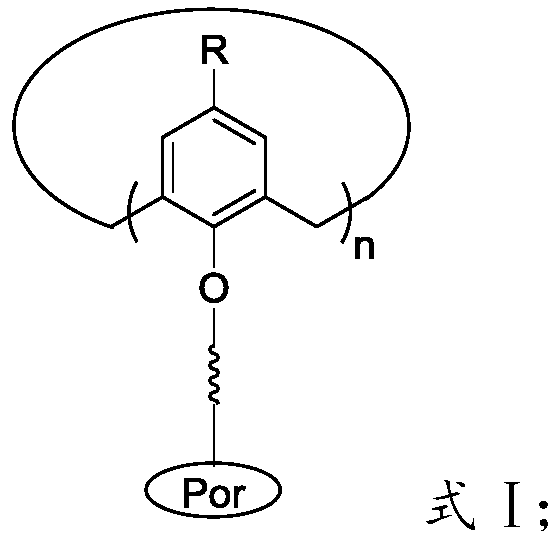
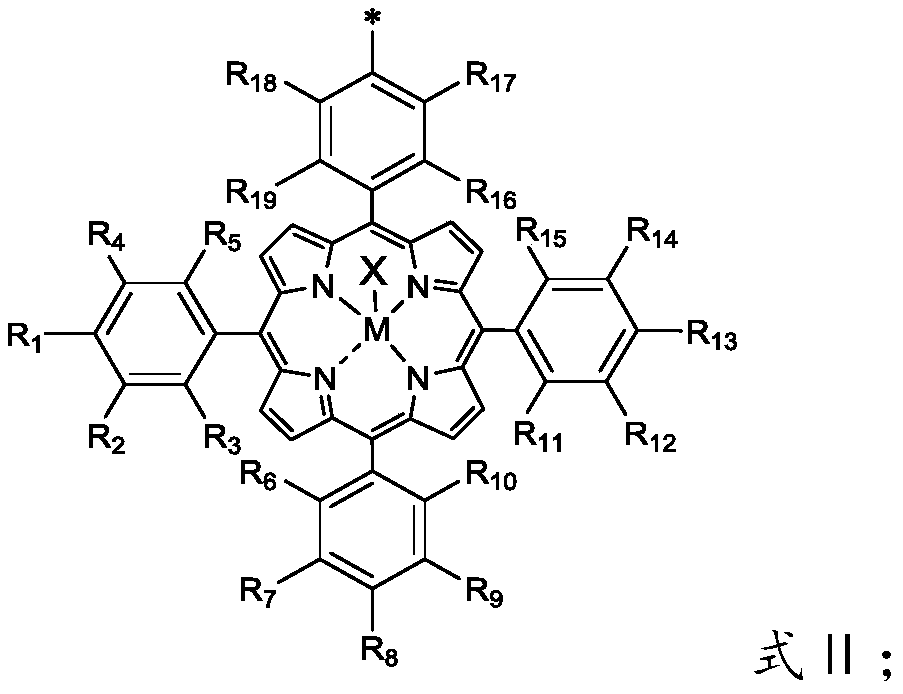
Macrocyclic carrier multi-center metalloporphyrin complex and preparation method of polycarbonate
A metalloporphyrin, multi-center technology, applied in the field of preparation of macrocyclic carrier multi-center metalloporphyrin complexes and polycarbonate, can solve problems such as being difficult to meet market demands
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0079] The present invention to the described The preparation method of is not particularly limited, and may be commercially available, or prepared according to methods well known to those skilled in the art.
[0080] Preferably in the present invention, the The specific preparation method is:
[0081] The p-hydroxybenzaldehyde, substituted benzaldehyde, and pyrrole were subjected to a "one-pot" reaction under the condition of propionic acid reflux, and the second color band was collected by column chromatography separation technology to obtain a monohydroxyl substituted asymmetric porphyrin; the central metal M and the co-ligated Body X is coordinated to the porphyrin ring through the metallation reaction of the porphyrin ligand in dichloromethane solution; The reaction to synthesize ethers takes place under neutral conditions.
[0082] said for linking groups.
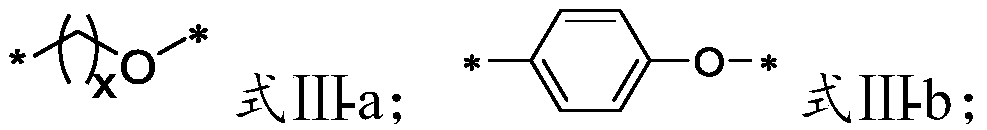
[0083] Preferably, the It has the structure shown in formula III-a or formula III-b:
[0084]
[008...
Embodiment 1
[0101]
[0102] A mixture of p-tert-butylphenol (4.0g, 26.6mmol), sodium hydroxide (NaOH) (1g) and formaldehyde (2.0ml, 71.2mmol) solution was placed in an open container in a microwave synthesizer (CEM) Irradiate with 100w power and stir for 3min. Cooling for 10 minutes gave a yellow solid. Add 4ml of toluene and 30ml of diphenyl ether, and then irradiate with 100W microwave power for 5 minutes under stirring to obtain a dark brown solution. This solution was added to 75 ml of ethyl acetate and allowed to stand for 2 hours. Filtration and washing with ethyl acetate, drying afforded the product EL1. The yield is about 80%. 1 HNMR: (DMSO, ppm) 1.1,3.8,7.1,9.7, high-resolution electrospray mass spectrometry analysis, analysis result is [C 44 h 56 o 4 ]: 648.42, found: 648.37.
[0103] Add 15g (120mmol) of 3-hydroxybenzaldehyde, 68.1g (370mmol) of 4-bromobenzaldehyde and 33g (490mmol) of pyrrole into 500mL of propionic acid, heat up to about 130°C, reflux for 1.5h, and ...
Embodiment 2
[0108]
[0109] 10 g (15.4 mmol) of EL1 and 8.8 g of phenol were dissolved in 200 mL of toluene. Add 16g (120mmol) of AlCl 3 , and the mixture was stirred at 60 °C for 6 h. After cooling, 200 mL of HCl (3%, v / v) was added, and the reaction mixture was stirred for 30 minutes. The aqueous phase was washed once with toluene, and the organic phase was washed with Na 2 SO 4 Dry, then remove toluene by rotary evaporation. 100mL CH 3 OH was added to the semi-solid residue. After filtration and drying, a white residue was obtained, which was washed with CHCl 3 / CH 3 Recrystallization from OH afforded 5.8 g of EL5 in about 87% yield. 1 H NMR (CDCl 3 , ppm): 10.2, 7.0, 6.7, 4.2, 3.5. High-resolution electrospray mass spectrometry analysis, the analysis result is [C 28 h 24 o 4 ]: 424.17, found: 424.09.
[0110] Under the protection of nitrogen, 2.03g (1.97mmol) EL3, 0.21g (0.49mmol) EL5, 0.12g potassium carbonate and 0.01g potassium iodide were dissolved in 200mL anhydr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com