Lactobacillus casei for prevention and auxiliary treatment of type II diabetes and application of lactobacillus casei
A technology of Lactobacillus casei and adjuvant therapy, which is applied in the field of microbiology and can solve problems such as drug dependence and drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The application provides a method for preparing probiotic compressed candy, the preparation method comprising: mixing and sieving the raw materials, and then compressing the tablets to obtain probiotic compressed candy, wherein the raw materials include bacteria powder of Lactobacillus casei, Skim milk powder, fruit powder, galacto-oligosaccharide powder, xylitol, microcrystalline cellulose and magnesium stearate.
[0034] In an achievable manner, the raw material mass ratio of the probiotic compressed candy is: 4-5 parts of bacteria powder, 20-30 parts of skim milk powder, 20-30 parts of fruit powder, 10 parts of galacto-oligosaccharide powder -15 parts, 4-5 parts of xylitol, 5-10 parts of microcrystalline cellulose, 0.5-1 part of magnesium stearate.
[0035] Put the bacteria powder of Lactobacillus casei according to the above mass ratio in the probiotic tablet candy, the number of viable probiotic bacteria is 10 9 CFU / g, the moisture content is less than 4%, and the...
Embodiment 1
[0040] Example 1: Isolation, identification and in vitro functional evaluation of Lactobacillus casei LCZ.
[0041] 1. Isolation and identification of Lactobacillus casei LCZ
[0042]Lactobacillus casei was isolated from the traditional kumiss in Xilin Gol Prairie, Inner Mongolia, and passed through MRS medium (Difco TM Lactobacilli MRS Agar), cultivated at 37°C for 72 hours, isolated a single colony, centrifuged to extract bacterial DNA, and then performed bacterial 16S universal primer PCR, and was identified as Lactobacillus casei by comparison of 16S rDNA.
[0043] For the Lactobacillus casei strain of the present application, a single colony is inoculated on the MRS solid medium, and it grows well aerobically at 37 ° C. The colony is round, with a diameter of 2.0-3.0 mm, the surface is not smooth, and it is milky white; Gram staining is positive , the thallus is relatively straight or slightly curved, with blunt ends, and exists in single or double.
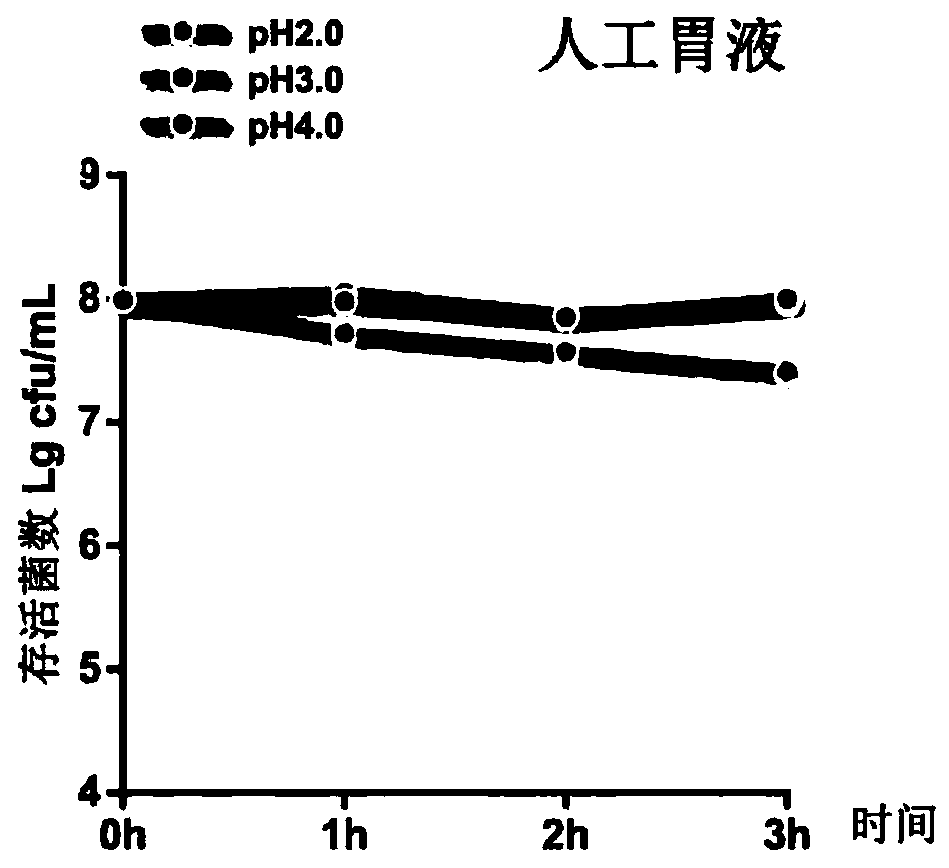
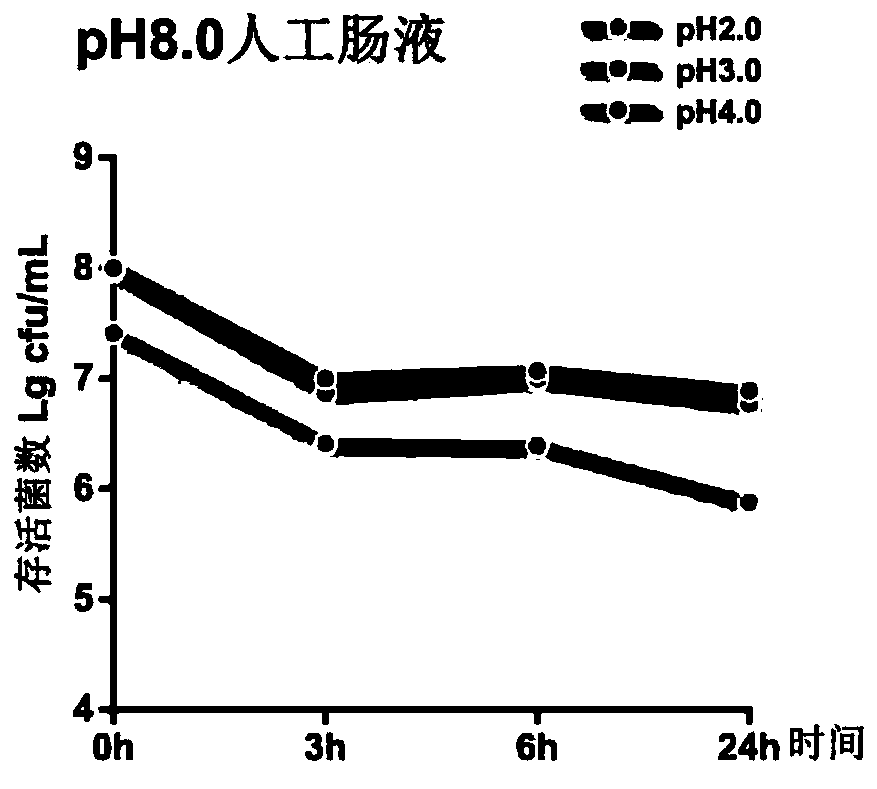
[0044] 2. Evalua...
Embodiment 2
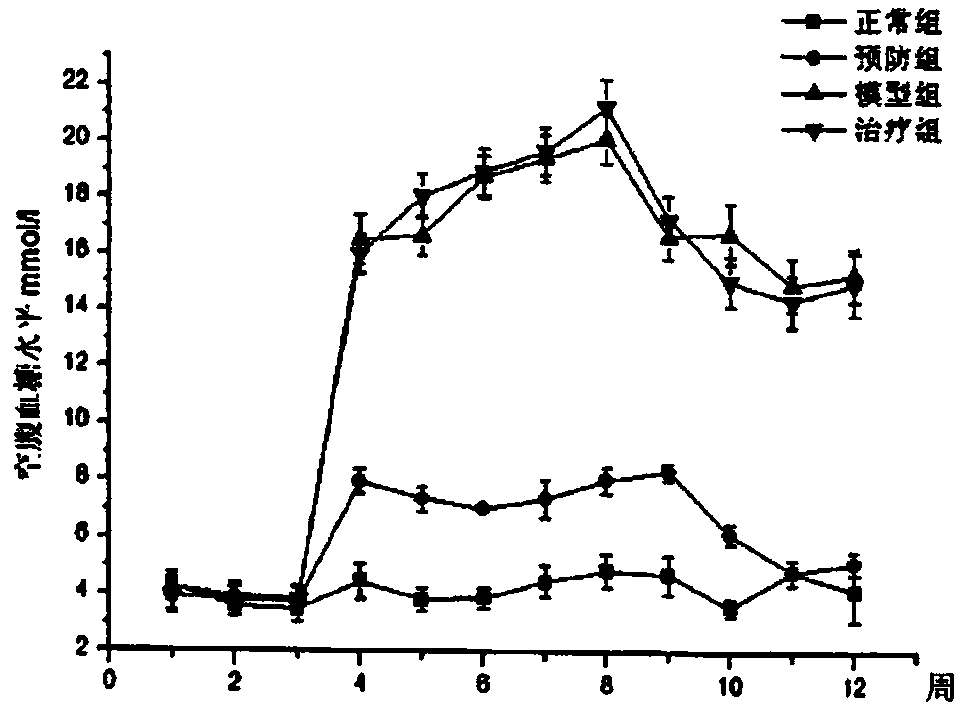
[0047] Example 2: Intervention of Lactobacillus casei LCZ for 2 weeks can well prevent the progress of type Ⅱ diabetes.
[0048] 8-week-old male SD rats were selected and divided into normal control group, prevention group, model group and treatment group. Except for the normal group, all other groups were fed with high-fat diet for 2 weeks, and then treated with STZ (streptozocin, streptozocin). ) induction to establish a type Ⅱ diabetes rat model. Among them, the prevention group self-administered Lactobacillus casei LCZ powder 4×10 9 cfu / g for 2 weeks. After modeling, the prevention group and the treatment group were intragastrically administered 5.0×10 9 cfu / g, each group was fed with normal feed for 8 weeks. Such as image 3 and 4 As shown, the fasting and postprandial blood glucose level indicators showed that the intake of Lactobacillus casei LCZ 2 weeks in advance had significant preventive effects on the fasting and postprandial blood glucose levels and prevent...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com