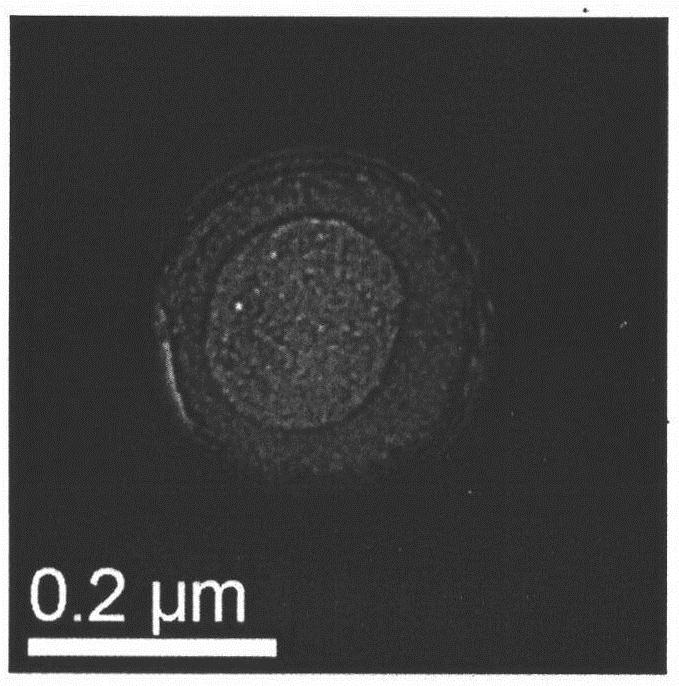
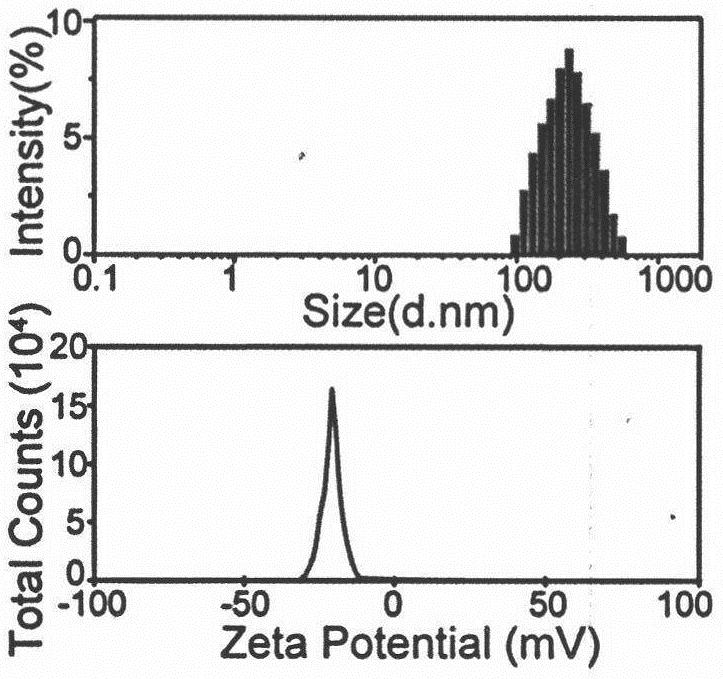
Nanoscale ultrasonic contrast agent entrapping perfluorocarbon
An ultrasonic contrast agent and perfluorocarbon technology, applied in the field of biomedical materials, can solve the problems of strong rigidity of inorganic nanoparticle materials, poor contrast agent stability, non-therapeutic damage, etc., to improve storage stability and ultrasonic response stability. The effect of stability, high security and strong penetration ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A method for preparing a nanoscale ultrasound contrast agent loaded with perfluorocarbons includes the following steps:
[0035] (1) Dissolve hydrogenated soybean phospholipids, PEGylated phospholipids and cholesterol in chloroform at a molar ratio, dissolve completely by ultrasonication, and evaporate under reduced pressure at 40°C to remove the organic solvent to obtain a lipid film. Add an appropriate amount of physiological saline as a hydration medium, and hydrate for 30 minutes at 37°C to obtain lipid vesicles.
[0036] (2) Take the liposome prepared in step (1), add perfluorocarbon, and conduct ultrasonic crushing with a probe at 50% strength, and obtain a coarse suspension after ultrasonication for 10 minutes.
[0037] (3) After mixing the coarse suspension prepared in step (2) with the lipid vesicles prepared in step (1), extrude through cellulose membranes of 400nm, 200nm, and 100nm in sequence, and the number of extrusions per layer No less than 13 times, ta...
Embodiment 2
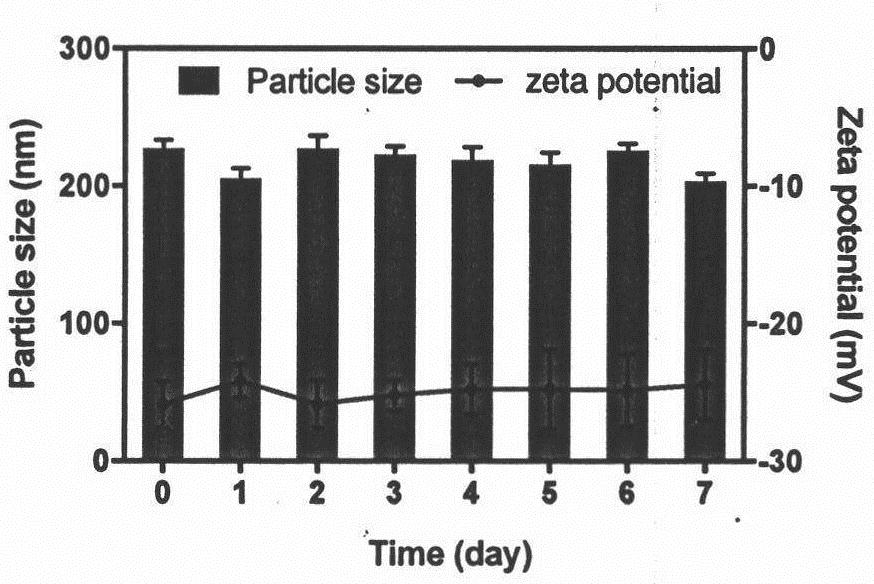
[0040] The storage stability evaluation of the ultrasound contrast agent prepared in Example 1 mainly includes the following steps:
[0041] The prepared contrast agent was placed at 4°C, and samples were taken on the 1st, 2nd, 3rd, 4th, 5th, 6th, and 7th day, and the particle size measurement and Zeta potential evaluation of the samples were carried out.
[0042] See the evaluation results image 3
Embodiment 3
[0044] The in vivo imaging effect evaluation of the ultrasound contrast agent prepared in Example 1 mainly includes the following steps:
[0045] (1) Male SD rats were selected, the abdominal hair was shaved, and barbiturates were injected intraperitoneally for anesthesia. Apply ultrasound coupling agent on the abdomen, and perform hepatic portal venography without contrast agent with ultrasound equipment, and see the appendix for the stored images Figure 4 .
[0046] (2) Dilute the contrast agent described in Example 1 5-10 times with normal saline, inject the contrast agent through the tail vein, and perform hepatic portal venography, and see the appendix for the stored image. Figure 5 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com