Preparation method and application of mimic enzyme MOF-based chiral separation material
A chiral separation and enzyme imitation technology, which is applied in the field of material chemistry and chiral chemistry, can solve the problems of expensive chiral ligands, and achieve the effects of wide separation of substrates, mild synthesis conditions and excellent selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] The preparation of enzyme-like MOF-based chiral separation materials, the reaction formula is as follows:
[0059]
[0060] The enzyme-like MOF-based chiral separation material is named compound 1. The above reaction formula is the smallest asymmetric unit of the crystal structure of compound 1, and its crystal structure is the periodic infinite extension structure of the asymmetric unit.
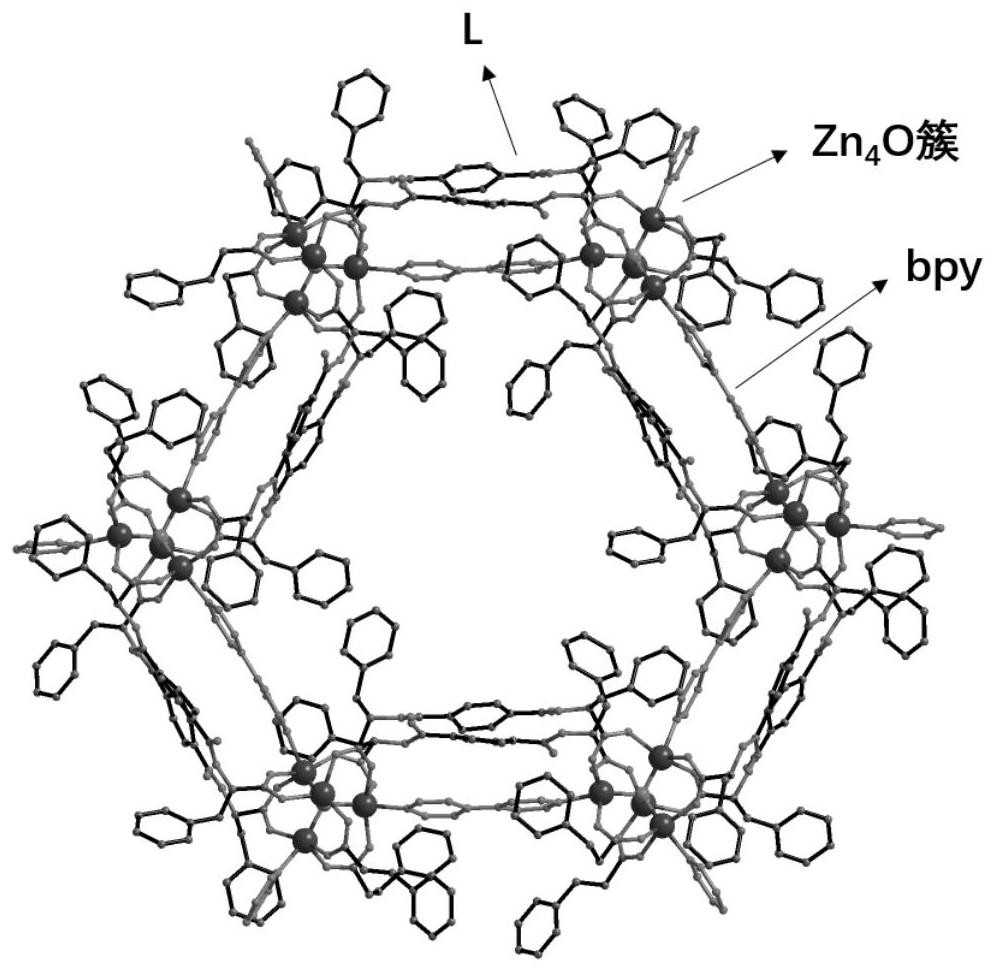
[0061] Zinc acetate dihydrate (18.3 mg, 0.10 mmol), monochiral ligand H 2 L (23mg, 0.05mmol) and auxiliary ligand bpy (7.8mg, 0.05mmol) were dissolved in N,N-dimethylacetamide (DMA), ethanol (EtOH) and water ( h 2 O) in the mixed solvent, the volume ratio of N,N-dimethylacetamide, ethanol and water is 1:1:1, stir evenly, and seal the resulting reaction liquid mixture in a 10mL explosion-proof vial. h 2 L is 2'2-(terephthaloyl bis(azadiyl)) bis(3-phenylpropionic acid), ligand H in the present embodiment 2 L is the existing technology, using Yu S L, Dou X Q, Qu D H, etal. -98. ...
Embodiment 2
[0064] Single crystal test and structural analysis of compound 1
[0065] The single crystal data of compound 1 is in the National Center for Protein Science Shanghai Synchrotron Radiation BL17B Multiple collections were carried out on the online station, and the optimal data were indexed, restored, and absorbed and corrected with the APEX3 software program. The final structure analysis and refinement were done manually through the SHELXS-97 program, during which the full-matrix least-squares refinement based on F 2 ) to determine all non-hydrogen atoms and perform anisotropic refinement. In addition, the hydrogen atoms on the ligand are completed by theoretical hydrogenation
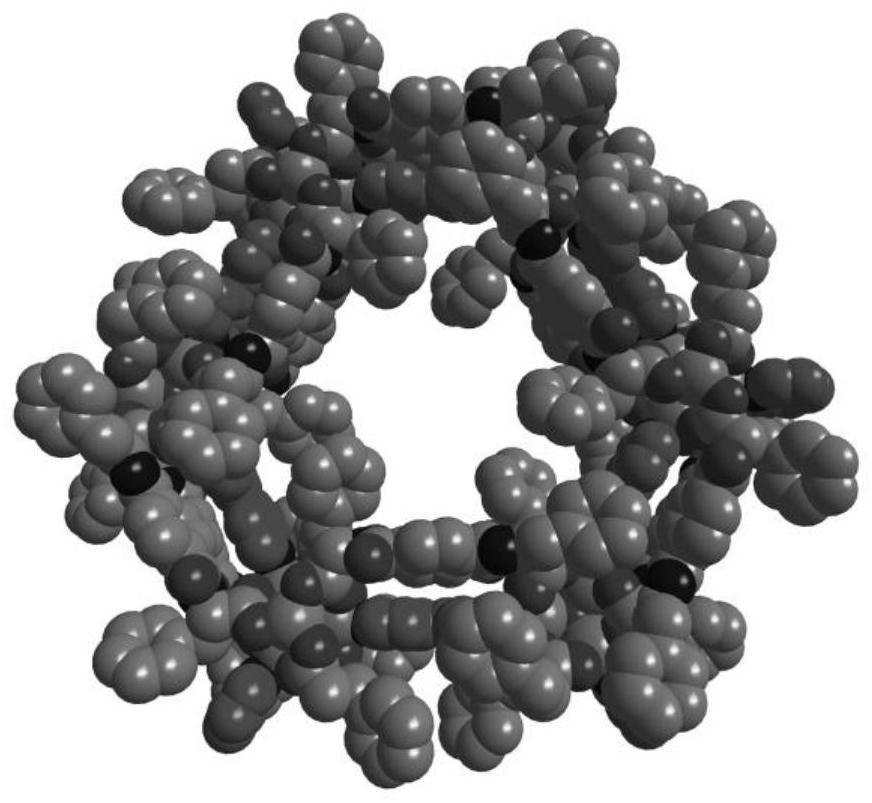
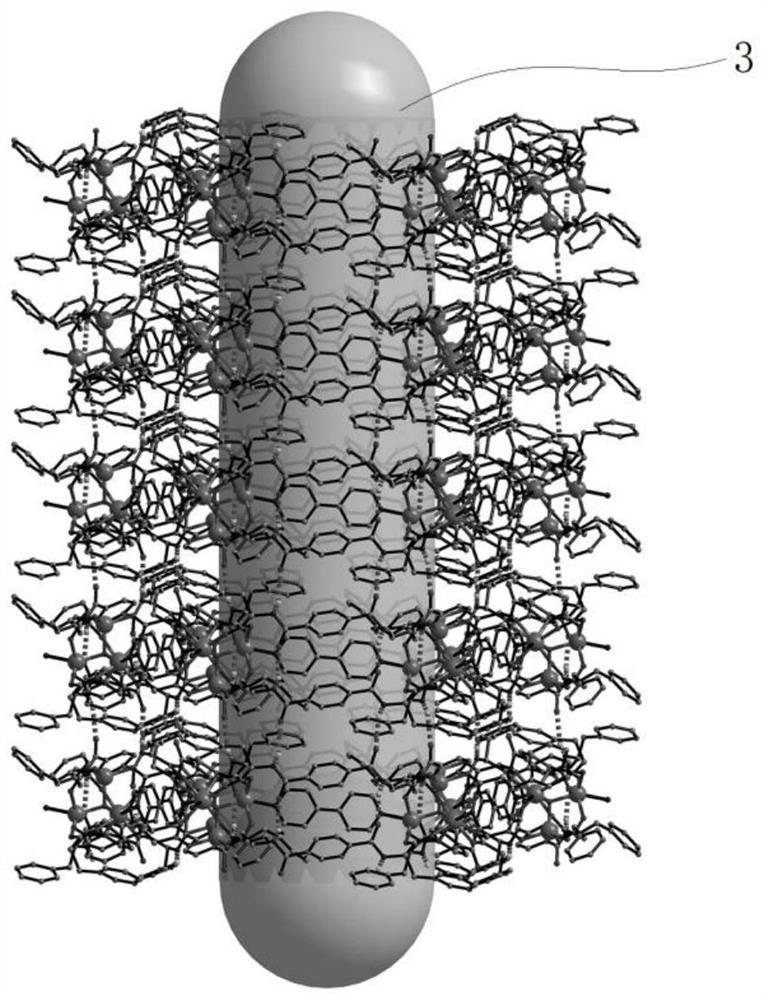
[0066] Such as Figure 1-Figure 4 As shown, single crystal diffraction data analysis showed that compound 1 crystallized in the chiral P32 1 Space group whose asymmetric unit content is its molecular formula [(Zn 4 O) 2 (L) 6 (bpy) 3 ], which contains 2 crystallographically independent Zn i...
Embodiment 3
[0070] The application of enzyme-like MOF-based chiral separation materials in the adsorption and separation of small molecular aromatic alcohols, the specific steps are as follows:
[0071] (1) Activation treatment: the crystal of compound 1 prepared in Example 1 was placed in a Soxhlet extractor, treated with anhydrous methanol for 24 hours, then treated with anhydrous acetone for 24 hours, and then activated in vacuum at 100°C to remove organic solvent;
[0072] (2) 50 mg of the crystal of compound 1 after the activation treatment was placed in a solution of racemic aromatic alcohol small molecule substrate (10 mg) in acetone (5 mL) to be resolved and allowed to stand for 5 h to allow the substrate to be fully adsorbed ; The above-mentioned crystals after fully adsorbing the chiral alcohol small molecule substrate are filtered, the racemic chiral alcohol small molecule substrate adhered to the surface of the compound 1 crystal is washed away with methanol, and the crystal o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com