G-17, PGI and PGII combined detection device and preparation method thereof
A joint detection and G-17 technology, applied in the direction of measuring devices, biological testing, material inspection products, etc., can solve the problems of insufficient protein adsorption and weak binding force of NC membrane, and achieve reasonable comprehensive judgment, simple operation, Practical effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
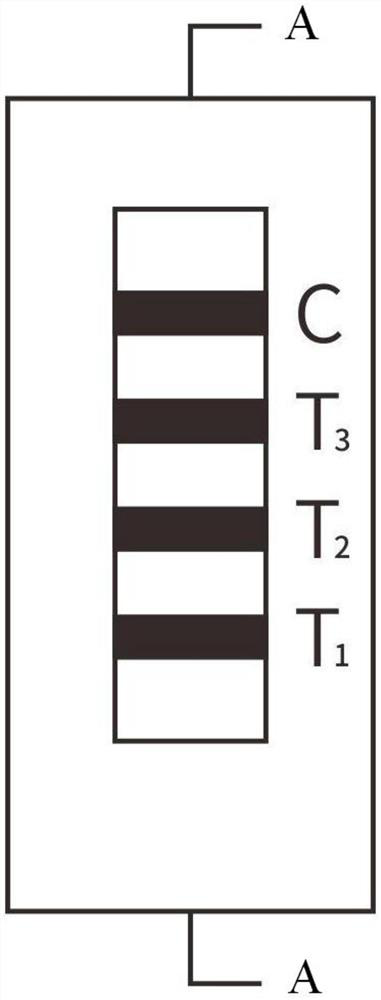
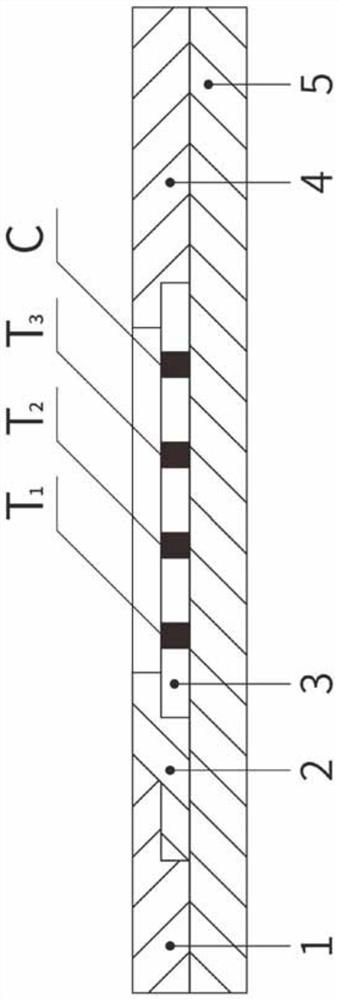
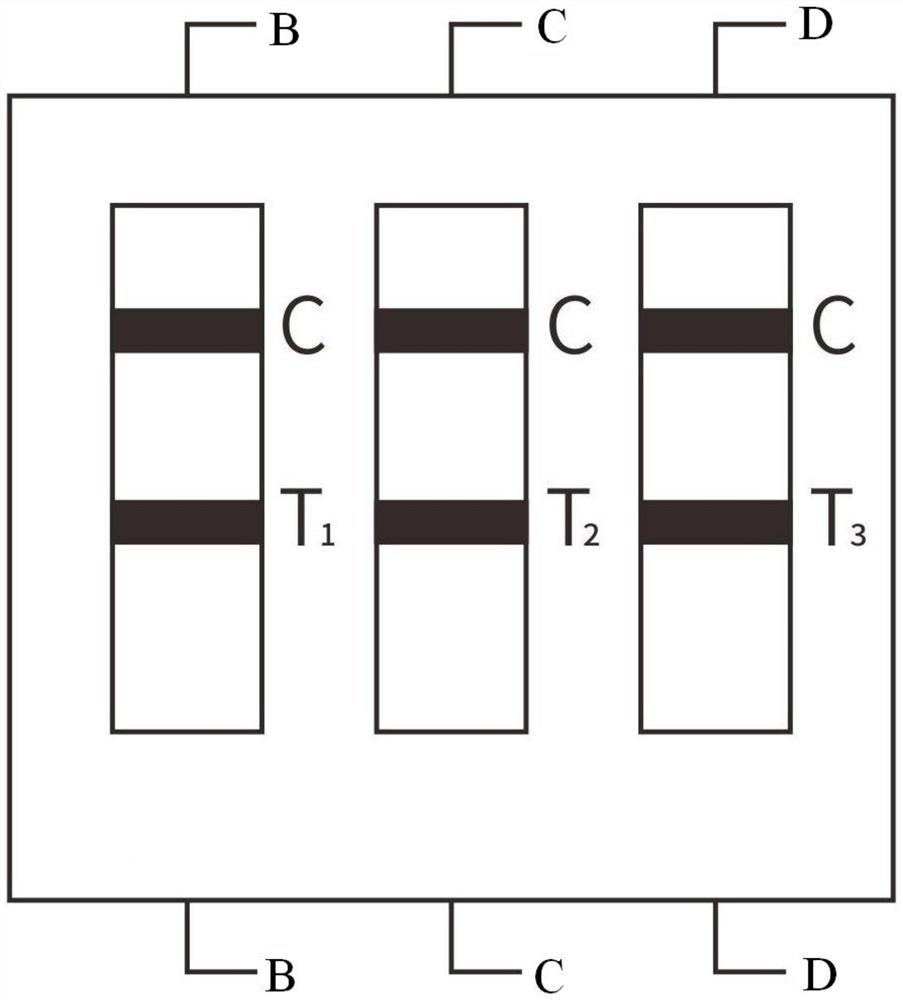
[0041] The sample pad 1, the immune colloidal gold glass fiber membrane 2, the immune nitrocellulose membrane 3, and the absorption pad 4 are pasted on the plastic plate 5 respectively, and the two ends of the immune nitrocellulose membrane 3 are respectively connected to the absorption pad 4, the immune colloidal gold glass The fiber membrane 2 is overlapped, and the other end of the immunocolloidal gold glass fiber membrane 2 is overlapped with the sample pad 1; the immunonitrocellulose membrane 3 is provided with a first detection line T1, a second detection line T2, and a third detection line. Line T3 and quality control line C; the first detection line T1 has a high-specificity gastrin 17 antibody on the solid phase; the second detection line T2 has a high-specificity pepsinogen I antibody on the solid phase; There is a highly specific pepsinogen II antibody on the solid phase of the third detection line T3; as figure 1 , 2 As shown, the detection lines T1, T2, and T3 ar...
Embodiment 2
[0067] Prepare colloidal gold particles with a size of 40nm;
[0068] Gold-spraying buffer solution includes: 20mM Tris-HCL solution, 12% sucrose concentration, 3% trehalose concentration, 0.7% BSA concentration, and 8.5 pH;
[0069] Preparation of polyethylene glycol glycerin treatment solution: mixed with polyethylene glycol glycerin and polylysine (SIGMA, 150KD), wherein the concentration of polyethylene glycol glycerin is 0.5%, and polylysine The concentration is 0.5%, filtered through a 0.22μm filter membrane, and set aside;
[0070] The sample pad treatment solution includes: the concentration of Tris-HCL solution is 0.1M, the concentration of bovine serum albumin BSA is 0.7%, the concentration of casein is 0.15%, and the concentration of surfactant is 0.7%;
[0071] All the other are with embodiment 1.
Embodiment 3
[0073] Prepare colloidal gold particles with a size of 60nm;
[0074] Gold-spraying buffer solution includes: 20mM Tris-HCL solution, 20% sucrose concentration, 5% trehalose concentration, 1% BSA concentration, and pH 8.5;
[0075] Preparation of polyethylene glycol glycerin treatment solution: mixed with polyethylene glycol glycerin, polylysine (SIGMA, 150KD) and PEG2000, wherein the concentration of polyethylene glycol glycerin is 0.5%, poly The concentration of lysine is 0.5%, and the concentration of PEG20000 is 0.1%, and it is filtered through a 0.22 μm filter membrane for later use.
[0076] The sample pad treatment liquid includes: the concentration of Tris-HCL solution is 0.1M, the concentration of bovine serum albumin BSA is 1%, the concentration of casein is 0.2%, and the concentration of surfactant is 1%;
[0077] All the other are with embodiment 1.
[0078] Further illustrate the effect of the present invention by experiment below.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com