A kind of fluorescent polylysine dendrimer, its preparation method and application
A technology of polylysine and fluorescent molecules, applied in the field of preparation of fluorescent polylysine dendrimers, can solve the problems of low contrast between tumors and normal tissues, low tumor specificity, short imaging time window, etc., and achieve Increased fluorescence lifetime and photostability, high purity, and accurate structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
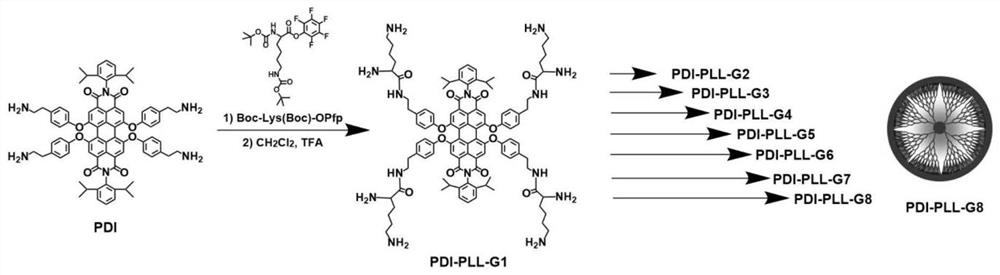
[0057] Embodiment 1: Synthesis of the first generation of fluorescent polylysine dendrimers (PDI-PLL-G1):
[0058] The molar ratio of PDI and Boc-Lys(Boc)-OPfp is 1:8.
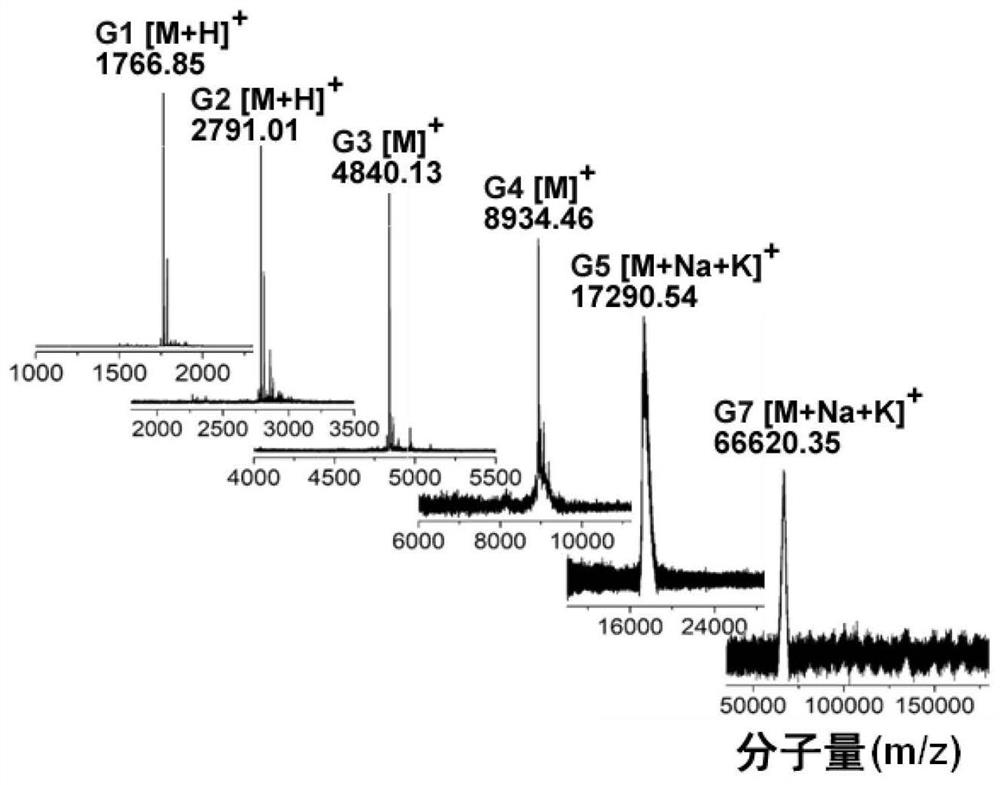
[0059] 1.00 g of PDI was dissolved in 30 mL of DMF, and then 0.45 g of DIPEA (1.5 times the equivalent of the amino group of PDI, to absorb the acid after deprotecting the amino group in PDI) was added dropwise. 2.4 g of pentafluorophenol-activated lysine (Boc-Lys(Boc)-OPfp) was dissolved in 20 mL of dichloromethane, added to the above solution, and reacted at room temperature under nitrogen atmosphere for 24 h. The solvent was removed by rotary evaporation and dried. Column chromatography (SiO 2 , n-hexane:ethyl acetate, v:v=4:1) to separate and purify the product, and obtain the 0.5th generation fluorescent polylysine dendrimer (PDI-PLL-G0.5) after drying. Then weigh 1.00 g of PDI-PLL-G0.5, add 30 mL of dichloromethane: trifluoroacetic acid (v:v = 1:1), and stir at room temperature for 2 h. The solution ...
Embodiment 2
[0060] Embodiment 2: the synthesis of the second generation fluorescent polylysine dendrimer (PDI-PLL-G2):
[0061] The molar ratio of the amino group of PDI-PLL-G1 to Boc-Lys(Boc)-OPfp is 1:2.
[0062] 1.00 g of PDI-PLL-G1 was dissolved in 30 mL of DMF, and then 0.58 g of DIPEA was added dropwise. 3 g of Boc-Lys(Boc)-OPfp was dissolved in 20 mL of dichloromethane, added to the above solution, and reacted at room temperature under nitrogen atmosphere for 24 h. The solvent was removed by rotary evaporation and dried. Column chromatography (SiO 2 , n-hexane: ethyl acetate, v:v=4:1) the product was separated and purified to obtain the 1.5th generation fluorescent polylysine dendrimer (PDI-PLL-G1.5). Take 1.00 g of PDI-PLL-G1.5, add 30 mL of dichloromethane: trifluoroacetic acid (v:v=1:1), and stir at room temperature for 2 h. The solvent was concentrated by rotary evaporation, and the residue was added dropwise to 50 mL of glacial ether, and the precipitate was washed three t...
Embodiment 3
[0063] Embodiment 3: the synthesis of the third generation fluorescent polylysine dendrimer (PDI-PLL-G3):
[0064] The molar ratio of the amino group of PDI-PLL-G2 to Boc-Lys(Boc)-OPfp is 1:2;
[0065] 1.00 g of PDI-PLL-G2 was dissolved in 30 mL of DMF, fully dissolved, and then 0.67 g of DIPEA was added dropwise. 7.2 g of Boc-Lys(Boc)-OPfp was dissolved in 20 mL of DMF, added to the above solution, reacted at room temperature for 24 h under a nitrogen atmosphere, and concentrated by rotary evaporation. The concentrated solution was added dropwise to glacial ether (the volume ratio of the concentrated solution to glacial ether was 1:5), the precipitate was washed 3 times, and dried to obtain the 2.5th generation fluorescent polylysine dendrimer (PDI-PLL-G2.5 ). Take 1.00g of PDI-PLL-G2.5, add 30mL of trifluoroacetic acid, and stir at room temperature for 6h. After the solution was concentrated, it was added dropwise to 50 mL of glacial ether, and the precipitate was washed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com