HPLC detection method of tramadol hydrochloride impurity E
A tramadol hydrochloride and detection method technology, applied in the field of drug analysis, can solve problems such as complex developing solvent system, uncontrollable data, poor retention of impurity E, etc., and achieve easy product quality control, high accuracy and precision , good stability and reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] (1) Instrument: Shimadzu liquid chromatograph LC-20AT;
[0056] Chromatographic column: Agilent ZORBAX SB-Phenyl 4.6×250mm 5μm;
[0057] Mobile phase: 0.2% trifluoroacetic acid-acetonitrile (83:17) is the mobile phase;
[0058] Wavelength: 270nm;
[0059] Column temperature: 40°C;
[0060] Flow rate: 1.0ml / min;
[0061] Injection volume: 20ul.
[0062] (2) Determination method:
[0063] Precisely measure 20 μl of each solution, inject it into a liquid chromatograph, record the liquid chromatogram, and calculate the peak area according to the external standard method.
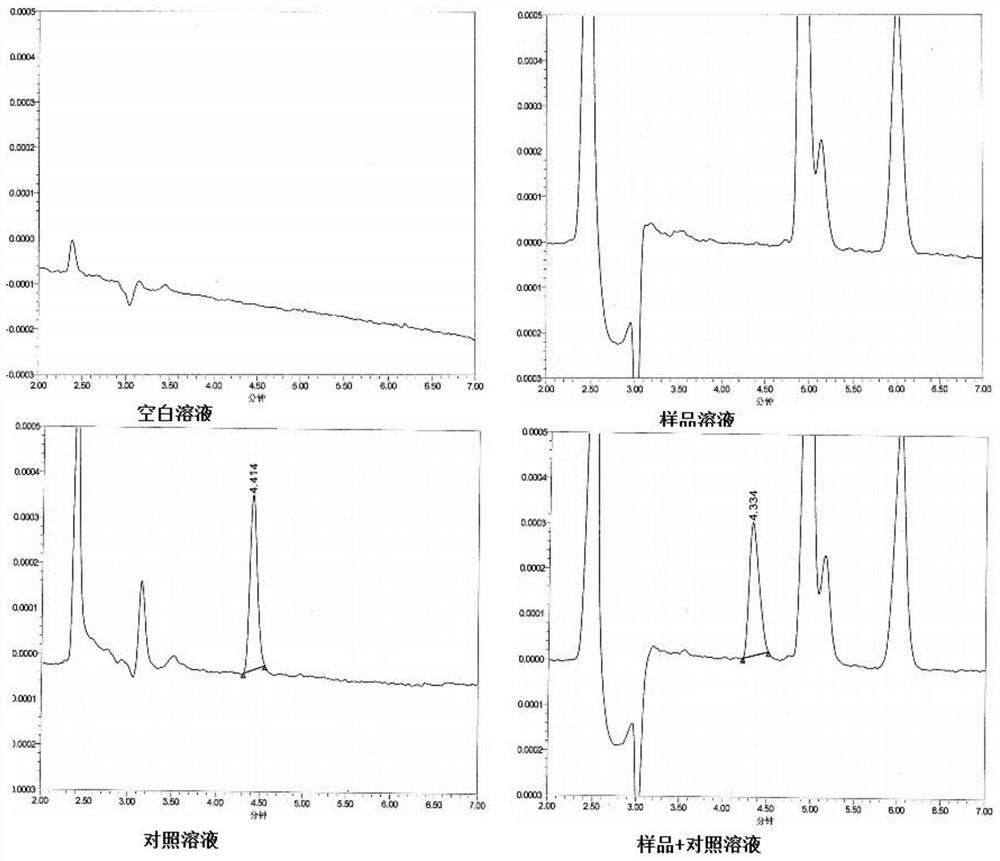
[0064] Table 1 Chromatographic peak positioning and resolution test results
[0065] Solution name name retention time (min) Peak area Reference solution Impurity E 4.414 2300 Spiked test solution Impurity E 4.334 2277
[0066] The results showed that the blank solution, the test solution and other impurities in the test did not interfere with the detection of im...
Embodiment 2
[0068] Implementation steps:
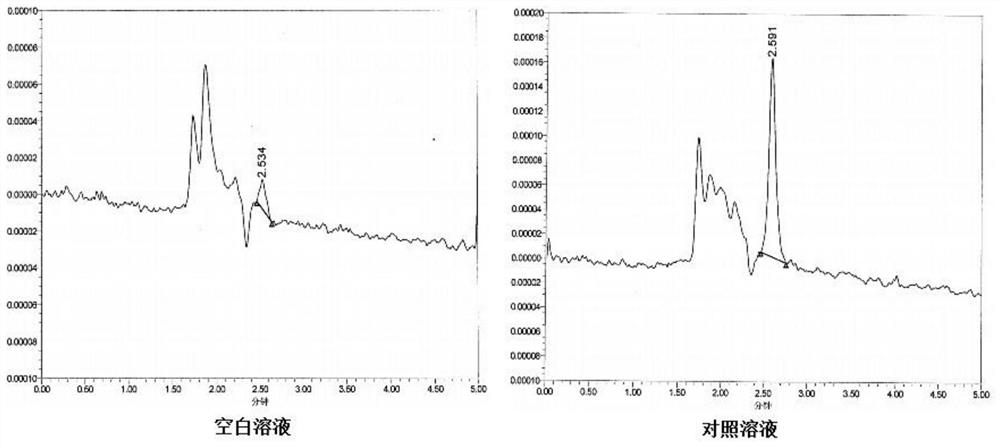
[0069] Solution preparation: Accurately weigh an appropriate amount of the impurity E reference substance, dissolve and dilute it with the mobile phase to make a solution with a concentration of about 200 μg / ml, and use it as the impurity E reference substance stock solution. Dilute step by step with the mobile phase to prepare a solution with a concentration of about 1 μg / ml, accurately measure 20 μl, inject it into the liquid chromatograph, record the chromatogram, and visually observe that the peak height of the impurity E chromatographic peak is about 3 times the baseline noise, which can be As the detection limit, the concentration of impurity E is about 1 μg / ml, which is equivalent to 0.005% of the concentration of the test solution;
[0070] Take the impurity E reference substance stock solution, dilute it step by step with the mobile phase, and prepare a solution with a concentration of about 2 μg / ml, accurately measure 20 μl, inject it i...
Embodiment 3
[0075] On the basis of the established chromatographic conditions, change the column temperature, change the flow rate, and change the ratio of the mobile phase, and accurately measure 20 μl each of the blank solvent and the spiked test solution, inject them into the liquid chromatograph, and record the chromatogram.
[0076] Table 3 Impurity E inspection durability test results
[0077]
[0078] The results showed that the separation between main peak and impurity, and between impurity and impurity was greater than 1.5 with small adjustment of column temperature and flow rate and replacement of chromatographic column, and the durability of the method was good. During the production process, slight changes in the detection environment will not affect the accuracy of the detection.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com