Monoclonal neutralizing antibody of HPV52L1 and application of monoclonal neutralizing antibody
A technology of monoclonal antibody, cgmccno.19186, applied in applications, antibodies, antiviral agents, etc., can solve the problems of limiting monoclonal antibody detection of HPV52 virus HPV52 virus treatment, etc., and achieve good sensitivity and specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] Embodiment 1, establishment of hybridoma cells
[0097] 1. Animal immunization
[0098] Using the Escherichia coli expression system, the virus packaging particles of the L1 protein of HPV52 type were prepared as the antigen. For the first immunization, the antigen was mixed with Freund’s complete adjuvant in equal volumes and fully emulsified, and injected subcutaneously in points. Each Balb / c mouse The injection volume was 100 μg, and 3 mice were immunized; on the 7th, 14th, and 21st days of the first immunization, the emulsion of antigen and Freund's incomplete adjuvant was used for booster immunization, and on the 14th day, small Blood was collected from the tail vein of the rat, the serum was separated, and the antibody titer was detected by indirect ELISA method. The antibody titer of the mouse serum was 1:16000, >1:32000, and 1:16000 respectively, and the No. 2 mouse with the highest titer was selected for fusion .
[0099] The operation steps of the indirect E...
Embodiment 2
[0108] Example 2: Preparation and identification of monoclonal antibodies
[0109] 1. Preparation of monoclonal antibody against HPV52 from mouse ascites
[0110] Adult BALB / c mice were selected, and hybridoma cells 52-1F5 were inoculated intraperitoneally, 1×10 per mouse 6 -2×10 6 First, when the abdomen is obviously enlarged and the skin feels tense when touched with hands, ascites can be collected with a 16-gauge needle. Centrifuge the ascites (13000r / min for 30 minutes), remove cell components and other precipitates, collect the supernatant, purify the antibody with a ProteinG affinity column, and detect the titer of the ascites and the purified antibody by indirect ELISA. The results show that the ascites was diluted 100,000 times positive, the purified antibody diluted to 1ng / mL is still positive.
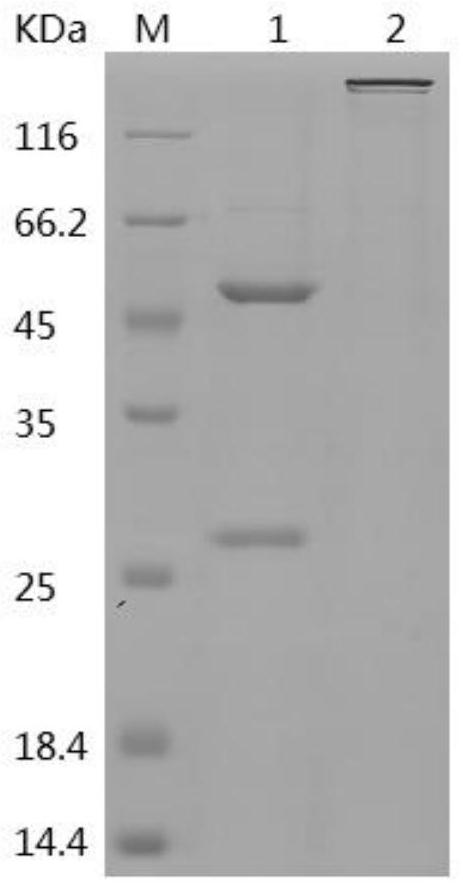
[0111] 2. Antibody Purity Determination
[0112] The purified antibody was subjected to 12% SDS-PAGE electrophoresis, and the results showed that the purity was above 95%...
Embodiment 3
[0120] Example 3: Detection of antibody neutralization activity
[0121] Through the pseudovirus-cell neutralization model, the neutralizing activity of the antibody was tested.
[0122]First dilute the antibody with PBS to 1000ng / mL, then dilute the antibody to 0.06ng / mL by 2-fold gradient, take 50μL of each concentration of antibody and 50μL of appropriate concentration of HPV6, 11, 16, 18, 31, 33, 35, 39, 45, 52, 58, 59, and 68 pseudoviruses were incubated in a 96-well plate at 4°C for one hour, and a negative serum control, a positive serum control, a cell control, and a pseudovirus control were set. Then each mixture was added to the 96-well plate pre-coated with 293FT cells and cultured in a cell culture incubator for 72 hours. Afterwards, the fluorescence was observed, and the cells were collected to detect the fluorescence by flow cytometry. If there was an inhibitory effect, the fluorescence inhibition rate was calculated according to the fluorescence inhibition rate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com