Homoserine lactonase mutant, coding gene and application of homoserine lactonase mutant in replacing antibiotics
A technology of homoserine lactone and serine lactone, applied in the field of agricultural biology, can solve the problems of poor enzyme stability and high use cost, achieve the effects of improving specific activity, reducing use cost, and meeting application and promotion needs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1 homoserine lactonase AIIA1 and mutants AIIA1-Y128H Cloning and expression vector construction
[0028] The present invention is derived from thermoacidophilic bacteria Alicyclobacillus acidoterrestris The homoserine lactonase (whose amino acid sequence is as shown in SEQ ID NO: 3) is the parent, which is expressed after replacing the Y128H site of the acidic homoserine lactonase using molecular biology techniques.
[0029] SEQ ID NO:3 is shown.
[0030] by Alicyclobacillus acidoterrestris Using genomic DNA as a template, the wild-type gene was first cloned and connected to the pET30a(+) expression vector to obtain AIIA1 -pET30a(+), with AIIA1 -pET30a(+) plasmid was used as a template, and the gene encoding a high specific activity homoserine lactonase mutant was amplified by over-lap PCR to construct AIIA1-Y128H - pET30a(+) expression vector.
Embodiment 2
[0031] Example 2 Preparation of Homoserine Lactonase Mutant with High Specific Activity.
[0032] Separately AIIA1 -pET30a(+) and AIIA1-Y128H - pET30a(+) expression vector was transformed into Escherichia coli competent cell BL21(DE3) to obtain a recombinant expression strain AIIA1 -pET30a(+)-BL21(DE3) and AIIA1-Y128H -pET30a(+)-BL21(DE3).
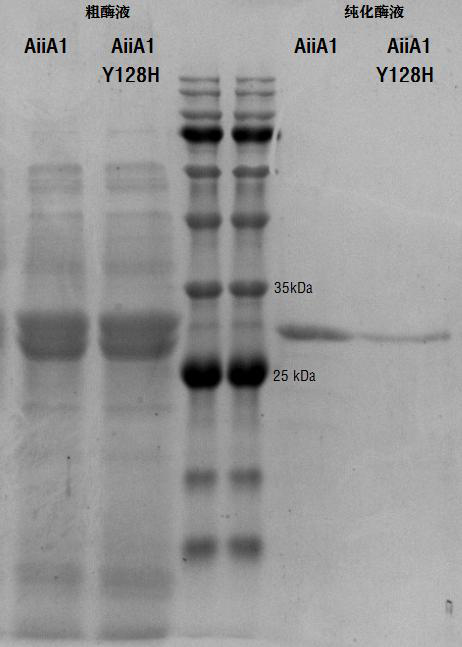
[0033] The constructed recombinant expression strain AIIA1 -pET30a(+)-BL21(DE3) and AIIA1-Y128H -pET30a(+)-BL21(DE3) was activated overnight, then transferred to a 1 L large bottle containing 300 ml LB culture medium (containing 50 μg / ml Kana) at 1%, and cultivated to OD at 37 °C and 220 rpm 600 When the value is about 0.6-0.8 (about 2-3 h), add a final concentration of 0.6 mM IPTG to induce expression, and transfer to 28 ° C, 190 rpm to induce culture for about 8-10 h. Collect bacteria by centrifugation at 12,000 rpm for 10 min. Bacteria were resuspended with 0.05mM crude enzyme solution at pH 7.0 and purified using His-tag p...
Embodiment 3
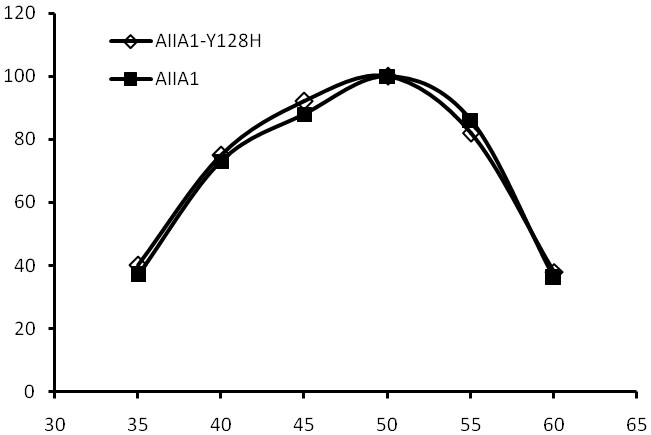
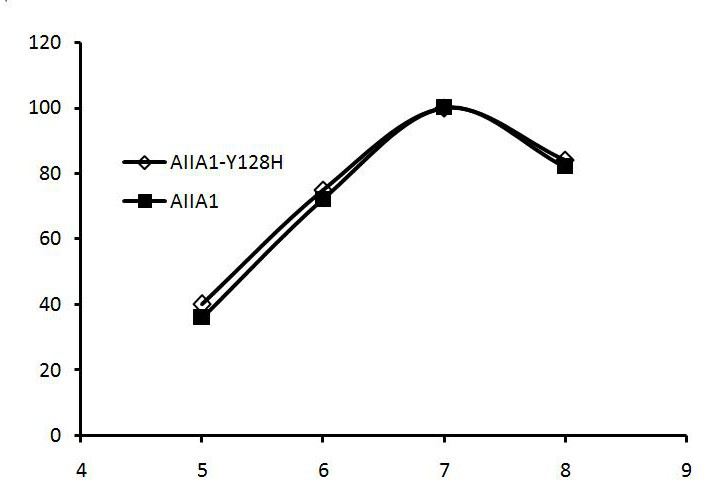
[0034] Example 3 Activity Analysis of Recombinant High Specific Activity Homoserine Lactonase Mutant and Wild Type
[0035] Enzyme activity was determined by HPLC method
[0036] (1) Principle of the method:
[0037] The quenching enzyme can break the lactone bond of N-acyl homoserine lactone to generate N-acyl homoserine, and the reduction of the substrate is proportional to the activity of the quenching enzyme in the reaction solution. Therefore, the activity of the quenching enzyme in the reaction solution can be calculated by measuring the reduction of the substrate by HPLC.
[0038] (2) Substrate solution: octanoyl homoserine lactone [3-oxo-C8-HSL] at a concentration of 0.1 mg / mL. Reaction system: draw 0.2ml of appropriately diluted enzyme solution, add 0.2ml of the above substrate, react at 30°C for 30 minutes, add 0.1ml of 10% SDS to terminate the reaction, and measure the remaining amount of the substrate.
[0039] (3) Mobile phase solution: 36% acetonitrile-water s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific vitality | aaaaa | aaaaa |
| Specific vitality | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com