Mitochondrion-lysosome migration type membrane potential fluorescent probe CSP
A fluorescent probe, membrane potential technology, applied in fluorescence/phosphorescence, luminescent materials, material analysis by optical means, etc., can solve the problems of limited detection sensitivity, automatic acquisition, practical operation and inconvenience of detection, etc., to achieve real-time Tracking, good reversibility effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
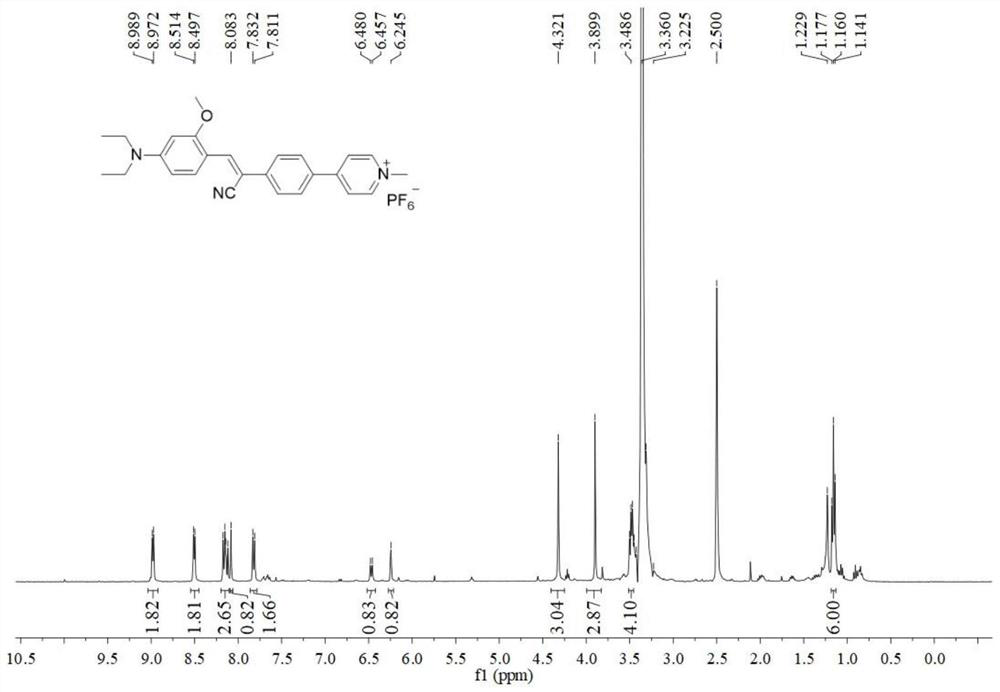
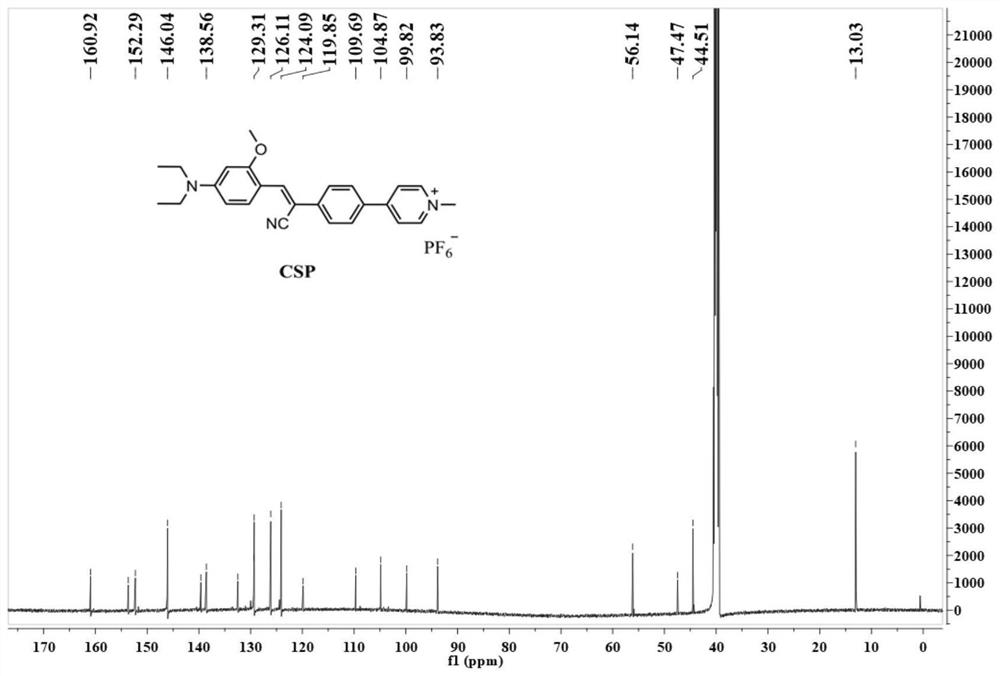
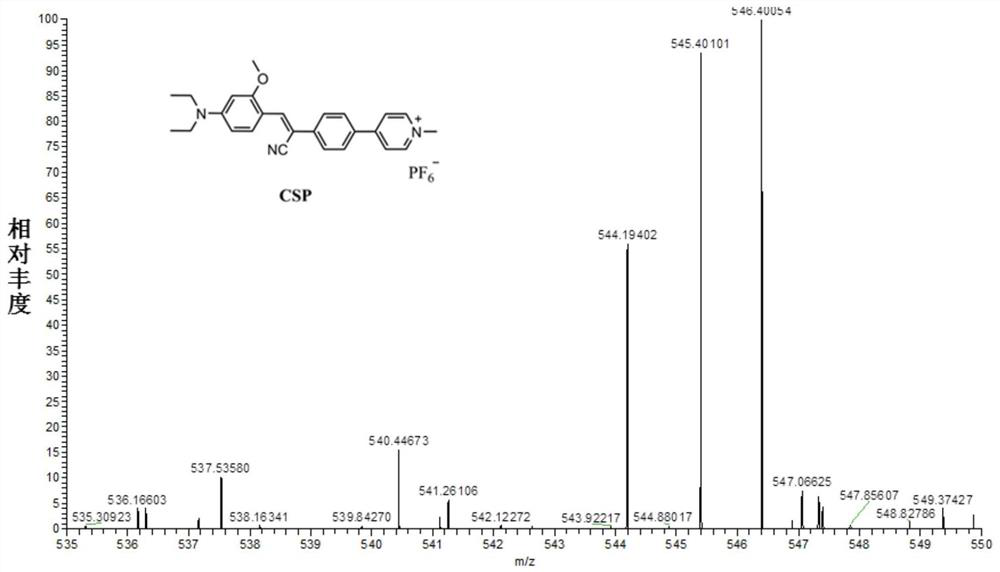
[0032] Preparation and characterization of a mitochondrial-lysosome migratory membrane potential fluorescent probe (CSP) with aggregation-induced emission properties:
[0033]
[0034] (1) In a round bottom flask, 4-bromophenylacetonitrile (0.588g, 3mmol) and t-BuOK (0.336g, 3mmol) were successively added to (30mL) absolute ethanol, and stirred at room temperature for 10 minutes; then 4-Diethylamino-2-methoxy-benzaldehyde (0.621g, 3mmol) was slowly added to the above mixture, and refluxed for 6 hours; the system was cooled to room temperature, the solvent was concentrated in vacuo, and purified by silica gel column chromatography (petroleum ether / ethyl acetate, 5:1, v / v) to obtain compound 2 (0.806 g, 70%) as a yellow solid. 1 H NMR (400MHz, CDCl 3 ):δ(ppm):8.26(d,J=8.8Hz,1H),7.91(s,1H),7.53–7.48(m,4H),6.36(dd,J=9.2,2.0Hz,1H),6.11 (s, 1H), 3.87 (s, 3H), 3.43 (q, J=6.8Hz, 4H), 1.23 (t, J=7.2Hz, 6H).
[0035] (2) Compound 2 (0.346g, 0.9mmol), K2 CO 3 (0.138g, 1mmol) and 4...
Embodiment 2
[0038] The near-infrared aggregation-induced fluorescence emission characteristics of the probe CSP in this example in acetonitrile / water mixed solvent:
[0039] The probe was diluted with acetonitrile / water mixed solvent to a final concentration of 5 μmol / L, the excitation wavelength was fixed at 475 nm, and the fluorescence emission spectrum of the probe changed with water content was recorded ( Figure 4 ), and plot the probe relative fluorescence intensity (I / I 0 ) in the acetonitrile / water mixed system with the curve of water content ( Figure 5 ). As the water volume ratio increased from 0% to 95%, the fluorescence intensity at 678nm increased sequentially, and reached the maximum when the water volume was 85%, indicating that the probe had typical aggregation-induced luminescence characteristics. Analysis of the standard by dynamic light scattering shows that the probe has formed an aggregated state with a hydration diameter of 135.1 nm in acetonitrile / water mixed sol...
Embodiment 3
[0041] The solid-state fluorescence emission performance of the probe CSP of this embodiment
[0042] The excitation wavelength was fixed at 475nm, and the solid-state fluorescence emission spectrum of the probe was recorded. Such as Figure 7 As shown, the maximum fluorescence emission intensity of the solid-state probe is 672nm, which is in the near-infrared emission range (fluorescence emission>650nm), indicating that the probe has aggregation-inducing properties of near-infrared emission. At the same time, the Stoke shift is as high as 197nm, which can effectively reduce the interference of excitation light. In addition, the solid-state probe exhibits bright red fluorescence under UV light (inset); the calculated solid-state fluorescence quantum yield of the probe is 9.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com