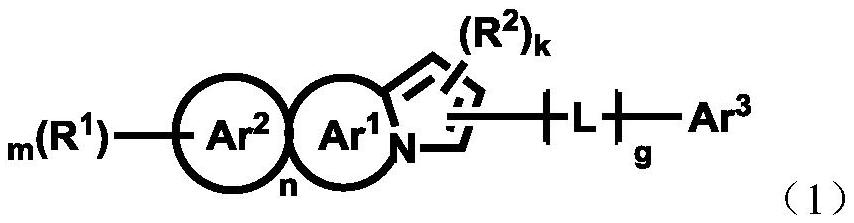
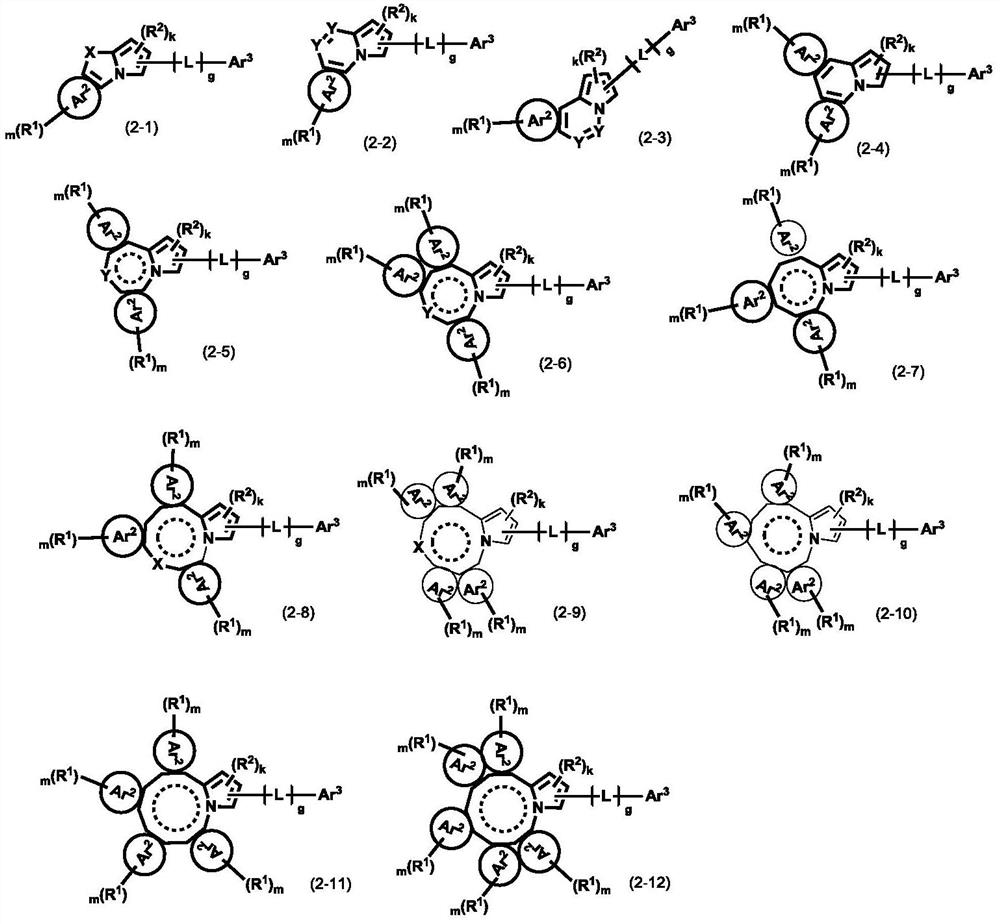
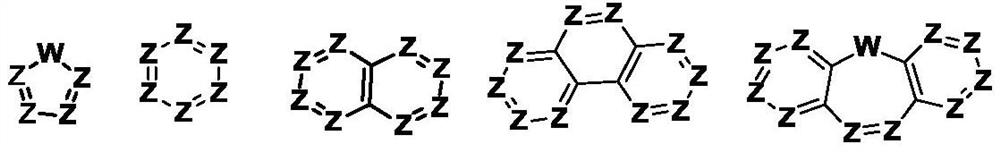
Pyrrolyl group-containing compound, polymer, mixture, composition, and organic electronic device
A compound, pyrrole-based technology, applied in the fields of polymers, compositions and their organic electronic devices, mixtures, and pyrrole-containing compounds, to achieve the effects of improving luminescence performance and lifespan, high external quantum efficiency, and long device lifespan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0169] Synthesis of compound (3):
[0170]
[0171] synthetic route:
[0172]
[0173] 1) Synthesis of intermediate 3-3: under nitrogen atmosphere, (22.3g, 100mmol) compound 3-1, (20.4g, 100mmol) compound 3-2, (9.55g, 50mmol) cuprous iodide, (5.7 g, 50mmol) trans-cyclohexanediamine, (31.8g, 100mmol) potassium phosphate and 250mL toluene were added to a 500mL three-neck flask, heated and stirred to 110°C for 12 hours, the reaction was completed, cooled to room temperature, and the filtrate was suction filtered , most of the solvent was evaporated by rotary evaporation, and washed three times with dichloromethane dissolved in water, and the organic phase was collected and mixed with silica gel to pass through a column for purification, and the yield was 75%.
[0174] 2) Synthesis of intermediate 3-4: under nitrogen atmosphere, add (18g, 60mmol) compound 3-3 and 150mL anhydrous tetrahydrofuran into a 500mL three-necked flask, cool down to -78°C, and slowly add 60mmol n-but...
Embodiment 2
[0181] Synthesis of compound (5):
[0182]
[0183] synthetic route:
[0184]
[0185] 1) Synthesis of intermediate 5-3: According to the synthesis method of intermediate 3-3, compounds 5-1 and 5-2 were substituted for compounds 3-1 and 3-2, and the yield was 75%.
[0186]2) Synthesis of intermediate 5-4: under nitrogen atmosphere, (11.3 g, 60 mmol) of compound 5-3 and 150 mL of triethyl phosphite were added into a 250 mL three-necked flask, heated at 160° C., and reacted for 12 hours. The reaction was stopped, and the liquid in the reaction solution was distilled out with a vacuum distillation device. The remaining solid was recrystallized with dichloromethane and ethanol solution, and the yield was about 80%.
[0187] 3) Synthesis of intermediate 5-5: under nitrogen atmosphere, (6.2g, 40mmol) compound 5-4, (8.2g, 40mmol) iodobenzene, (3.8g, 20mmol) cuprous iodide, (2.3g, 20mmol) trans-cyclohexanediamine, (12.7g, 40mmol) potassium phosphate and 100mL toluene were add...
Embodiment 3
[0192] Synthesis of compound (9):
[0193]
[0194] synthetic route:
[0195]
[0196] 1) Synthesis of intermediate 9-2: according to the synthesis method of intermediate 3-3, compounds 5-1 and 9-1 were substituted for compounds 3-1 and 3-2, and the yield was 70%.
[0197] 2) Synthesis of intermediate 9-3: (12.3g, 60mmol) compound 9-2, (17g, 120mmol) phosphorus pentoxide and 60mL trifluoromethanesulfonic acid were added to a 150mL three-necked flask, stirred at room temperature for 24 hours, To finish the reaction, slowly invert the reaction solution in 300°C of ice water, filter with suction, wash the filter residue with water, aqueous sodium bicarbonate solution, and water several times, collect the filter residue, dry it, place it in 50mL of pyridine, reflux for 12 hours, and cool After reaching room temperature, most of the solvent was evaporated by rotary evaporation, and extracted three times with dichloromethane, and the organic phase was collected and mixed with...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com