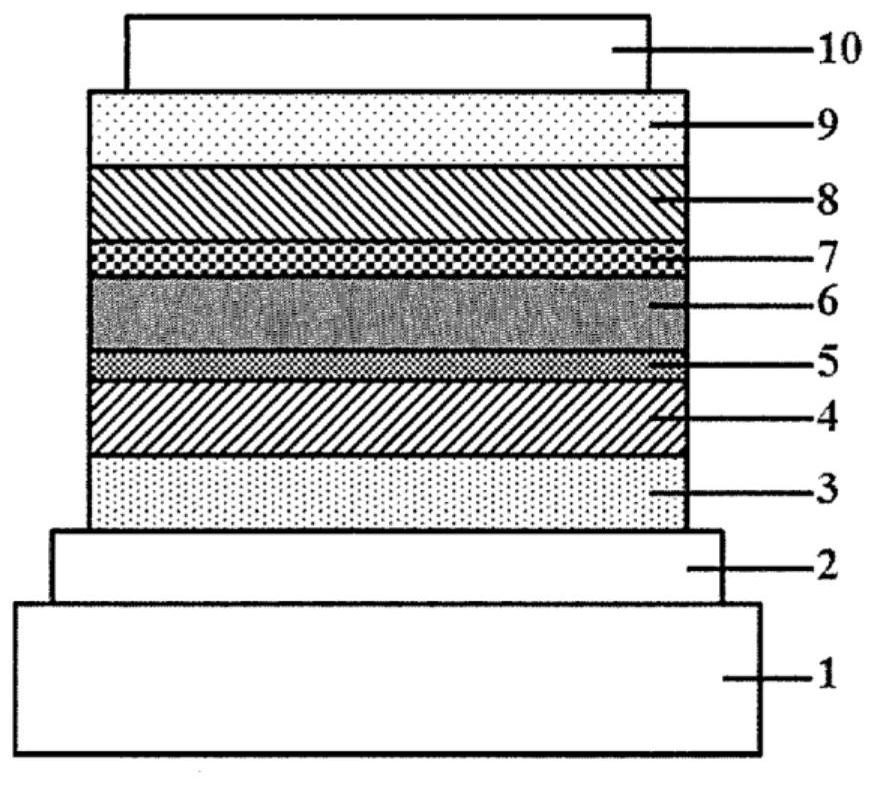
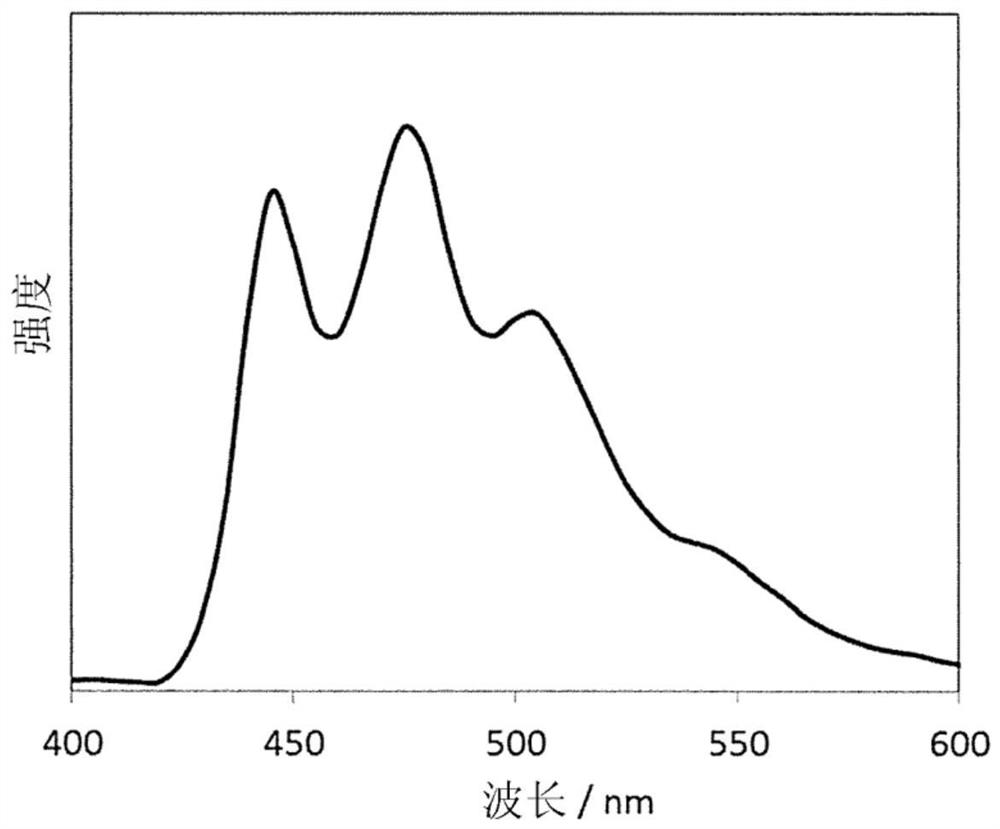
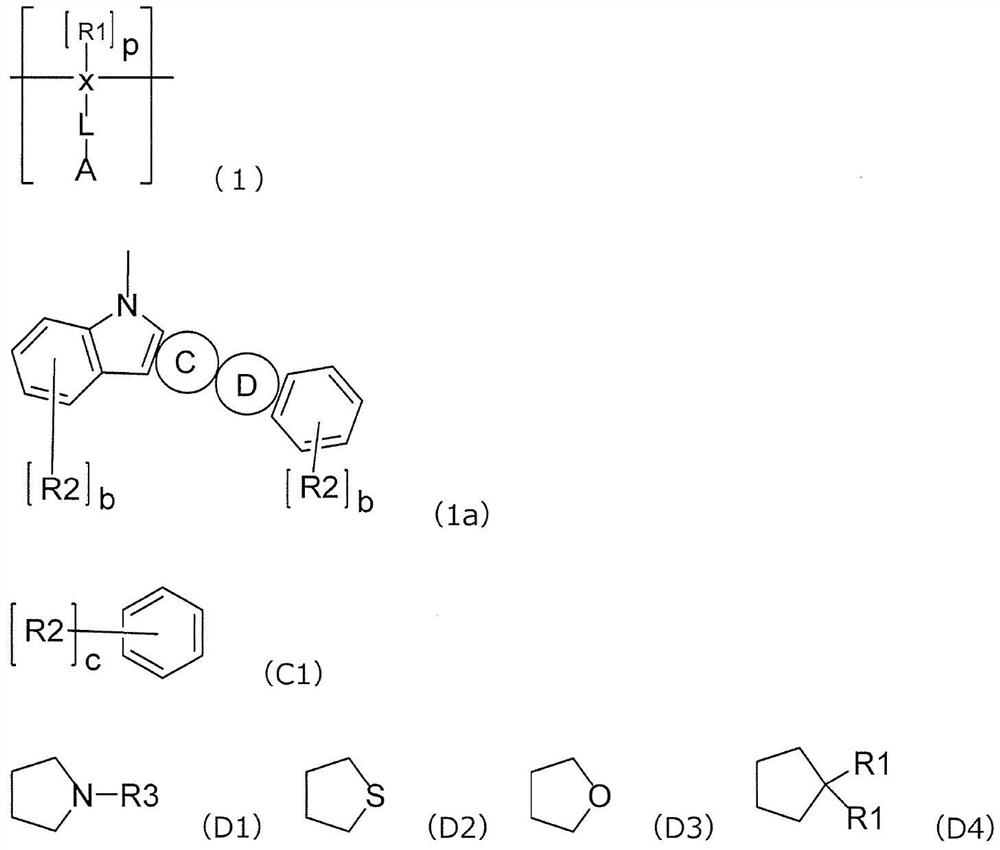
Polymer for organic electroluminescent element and organic electroluminescent element
A technology of electroluminescent components and polymers, applied in the direction of electroluminescent light sources, electrical components, electric light sources, etc., can solve the problems of insufficient efficiency and durability characteristics, and achieve high charge transfer characteristics, high driving stability, high performance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0145] Hereinafter, the present invention will be described in more detail based on examples, but the present invention is not limited to these examples, and can be implemented in various forms as long as the gist thereof is not exceeded.
[0146] Molecular weight and molecular weight distribution determination of polymers
[0147] The molecular weight and molecular weight distribution of the synthesized polymer were measured using GPC (manufactured by Tosoh, HLC-8120GPC), solvent: tetrahydrofuran (THF), flow rate: 1.0 ml / min, column temperature: 40°C. The molecular weight of the polymer was calculated as a polystyrene-equivalent molecular weight using a calibration curve based on monodisperse polystyrene.
[0148] Solubility Evaluation of Polymers
[0149] The solubility of the synthesized polymer was evaluated according to the following method. It was mixed with toluene so as to have a concentration of 0.5 wt%, and ultrasonic treatment was performed at room temperature for...
Synthetic example 1
[0152] Synthesis of Polymer A via Intermediates A, B, Polymerization of Intermediates A, B.
[0153] (Synthesis of Intermediate A)
[0154]
[0155] Under a nitrogen atmosphere, 5.13g (20.0mmol) of 11,12-indolinone[2,3-a]carbazole, 7.97g (20.0mmol) of 9-(3-biphenyl)-3-bromocarbazole were added ), 6.36 g (100.1 mmol) of copper, 8.30 g (60.0 mmol) of potassium carbonate, 653.0 mg (0.2 mmol) of 18-crown ether, and 60 ml of dimethylimidazolinone were stirred. Then, it heated to 190 degreeC, and stirred for 48 hours. After cooling the reaction solution to room temperature, copper and inorganic substances were filtered off. 200 ml of a mixed solvent of water:ethanol=1:1 was added to the filtrate, stirred, and the precipitated solid was filtered out. After drying this under reduced pressure, it was purified by column chromatography to obtain 9.41 g (16.4 mmol, yield 82.0%) of intermediate A as a white powder.
[0156] (Synthesis of Intermediate B)
[0157]
[0158] Under a...
Synthetic example 2
[0165] Polymer B was synthesized via Intermediates C, D and Intermediates E, F, Polymerized Intermediates C, D.
[0166] (Synthesis of Intermediate C)
[0167]
[0168] Under a nitrogen atmosphere, 5.13 g (20.0 mmol) of 11,12-indolinone [3,2-a] carbazole, 6.19 g (20.0 mmol) of 3-bromo-terphenyl, and 0.11 g of copper iodide were added. g (0.60mmol), tripotassium phosphate 21.24g (100.1mmol), trans-1,2-cyclohexanediamine 0.91g (8.01mmol), 1,4-di 100ml of alkane was stirred. Then, it heated to 130 degreeC, and stirred for 48 hours. After cooling the reaction solution to room temperature, inorganic substances were filtered off. The filtrate was dried under reduced pressure, and purified by column chromatography to obtain 9.10 g (18.8 mmol, yield 93.8%) of intermediate C as a white powder.
[0169] (Synthesis of Intermediate D)
[0170]
[0171] Under a nitrogen atmosphere, add intermediate C4.85g (10.0mmol), 1,3-dibromo-5-iodobenzene 3.62g (10.0mmol), copper iodide 0.0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com