Fluorescent probe for detecting sulfur dioxide derivative, detection object, and preparation method and application of fluorescent probe
A fluorescent probe, sulfur dioxide technology, applied in the field of applied biology, can solve problems such as poor water solubility, and achieve the effects of high sensitivity, low false positives, and fast response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The reaction scheme of the preparation method of fluorescent probe (i.e. probe DQ) of the present invention is as follows:
[0039]
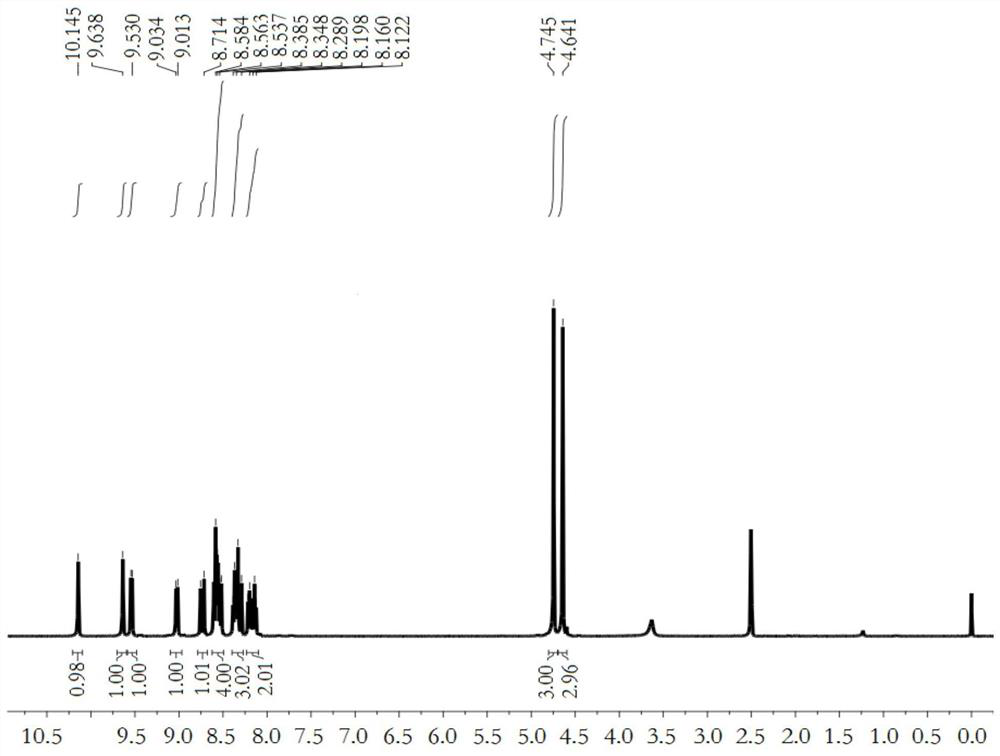
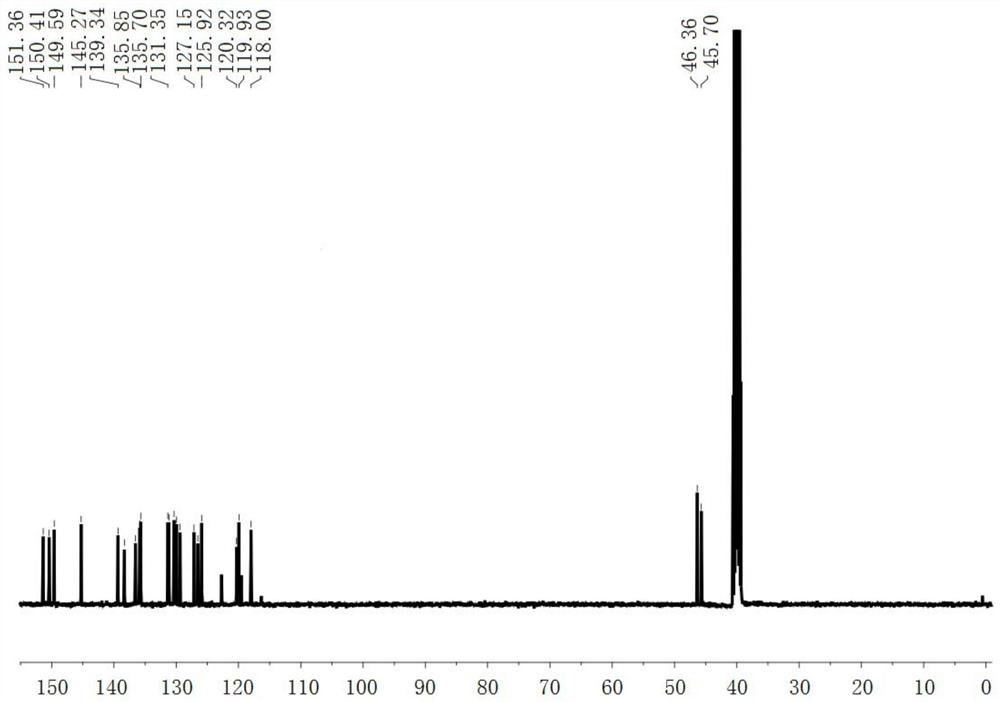
[0040] Compound 1 (212 mg, 0.5 mmol) and methyl trifluoromethanesulfonate (164 mg, 1 mmol) were dissolved in 10 mL of chloroform, the solution was stirred overnight at room temperature, and filtered with suction to obtain compound DQ (223 mg, yield 76%). Such as Figure 1-2 as shown, 1 H NMR (400MHz, DMSO-d 6 )δ(ppm): 10.15(s, 1H), 9.64(s, 1H), 9.54(d, J=6.4Hz, 1H), 9.02(d, J=8.4Hz, 1H), 8.73(d, J= 16.4Hz,1H),8.61-8.52(m,4H),8.39-8.29(m,3H),8.22-8.12(m,2H),4.75(s,3H),4.64(s,3H); 13 C NMR (100MHz, DMSO-d 6 )δ(ppm): 151.4, 150.4, 149.6, 145.3, 139.3, 138.3, 136.6, 135.9, 135.7, 131.4, 131.2, 130.4, 130.0, 129.4, 127.2, 126.5, 125.9, 120.5, 119.9, 167.4.
Embodiment 2
[0041] Embodiment 2 Probe DQ is to the absorption spectrum of bisulfite radical response and the fluorescence spectrum to sulfite radical / bisulfite radical
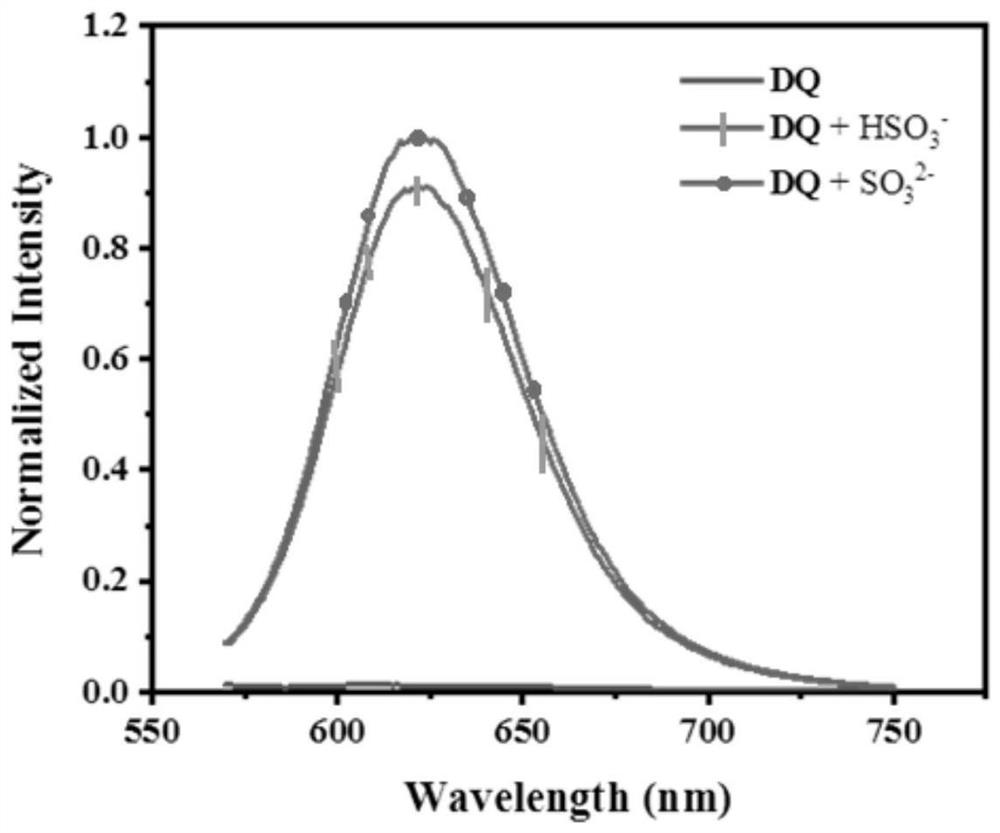
[0042] The probe compound DQ was dissolved in PBS solution (20 mM, pH 7.4) to form a 1 mmol / L solution. Add PBS buffer (20 mM, pH 7.4) to the pipetted probe DQ solution, and dilute to 10 μM (20 mM, pH 7.4) of the test solution. Add sulfite or bisulfite to the solution to be tested, and test the change of its fluorescence emission spectrum with a fluorescence spectrum and an ultraviolet spectrometer. The reaction mechanism is as follows: Figure 11 shown. Depend on image 3 It can be seen that the probe DQ has no fluorescence emission at 620nm, and after adding sulfite or bisulfite, the probe has a strong fluorescence emission peak at 620nm, which shows that sulfite or bisulfite can cause probe Enhancement of needle fluorescence emission. Depend on Figure 4 It can be seen that the fluorescence emission of the probe s...
Embodiment 3
[0043] Embodiment 3 Probe DQ is to the time research of bisulfite radical
[0044] The kinetics of the action of DQ on bisulfite was explored by testing the change of fluorescence intensity of probe DQ in response to bisulfite. Depend on Figure 7 It can be seen that after adding bisulfite to the probe DQ solution, the fluorescence intensity gradually increases with time, and the fluorescence intensity can reach the maximum within 15 seconds, indicating that the probe DQ can quickly detect bisulfite ions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com