Indolizine derivative and preparation method thereof
A derivative, indolazine technology, applied in the field of indolizine derivatives and their preparation, can solve the problems of complex catalyst preparation, unreusable, difficult product separation, etc., and achieves no catalyst residue, reduced burden, and atom economy. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
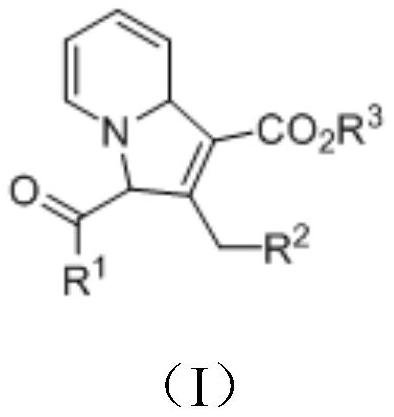
[0037] A kind of preparation method of the indoxazine derivative of formula (I) structure, comprises the following steps:
[0038] Under nitrogen atmosphere, mix the compound 1 with the structure of formula (II), the compound 2 with the structure of formula (III), the catalyst and the accelerator in the reaction solvent, and carry out the [3+2] cycloaddition reaction under stirring , to obtain indolezine derivatives;
[0039]
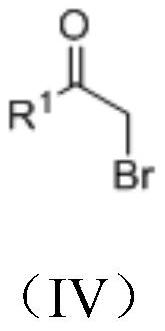
[0040] In formula (II), R 1 Be any in phenyl, substituted phenyl, substituted heteroaryl; In formula (III), R 2 is phenyl, substituted phenyl, hydrogen, benzyl, C 1 -C 20 Any of the alkyl groups; R 3 is an alkyl or aryl group.
[0041] The reaction formula of [3+2] cycloaddition reaction is as follows:
[0042]
[0043] Wherein, the molar ratio of compound 1 and compound 2 is 1:(1-3).
[0044] The above-mentioned catalyst is an inorganic base, and the accelerator is a Lewis base.
[0045] Wherein, the inorganic base is at least one of pota...
Embodiment 1
[0061] A kind of indoxazine derivative, the structure is Prepared by:
[0062] 1) Preparation of 1-(2-oxo-2-arylethyl)pyridinium bromide
[0063] Pyridine (2.0 times the amount of a-bromoacetophenone) and a-bromoacetophenone were stirred in ether solution for 2 hours, a large amount of solid precipitated, then filtered, washed with ether, and dried to obtain white pyridinium bromide salt. The reaction formula is as follows:
[0064]
[0065] 2) Preparation of butadienoate
[0066] Dissolve 10mmol of alkoxyacylmethyl triphenylphosphine bromide in 50ml of dichloromethane, then add 22mmol of triethylamine, after 1 hour of reaction, slowly add 11mmol of acid chloride dropwise into the mixed system, and wait for 24 After 1 hour, the solvent was removed, extracted with ether, spin-dried, and separated by column chromatography (ethyl acetate:petroleum ether=1:40) to obtain the obtained product. The specific reaction is as follows:
[0067]
[0068] 3) Preparation of Indox...
Embodiment 2
[0073] A kind of indoxazine derivative, structure is identical with embodiment 1, is prepared by the following method:
[0074] 1) Preparation of 1-(2-oxo-2-arylethyl)pyridinium bromide
[0075] Pyridine (1.0 times the amount of a-bromoacetophenone) and a-bromoacetophenone were stirred in ether solution for 2 hours, a large amount of solid precipitated, then filtered, washed with ether, and dried to obtain white pyridinium bromide salt.
[0076] 2) Preparation of butadienoate
[0077] Dissolve 10mmol of alkoxyacylmethyl triphenylphosphine bromide in 50ml of dichloromethane, then add 20mmol of triethylamine, after 1 hour of reaction, slowly add 10mmol of acid chloride dropwise to the mixed system, and wait for 24 After 1 hour, the solvent was removed, extracted with ether, spin-dried, and separated by column chromatography (ethyl acetate:petroleum ether=1:40) to obtain the obtained product.
[0078] 3) Preparation of Indoxazine Derivatives
[0079] Under a nitrogen atmospher...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com