Triphenylamine-triphenylphosphine compound as well as preparation method and application thereof
A technology of triphenylphosphine and triphenylamine, which is applied in the field of triphenylamine-triphenylphosphine compounds and their preparation, can solve the problem of insufficient anti-cancer effect, achieve excellent luminescent properties and prolong the triplet lifetime
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] A kind of triphenylamine-triphenylphosphine compound name is 4-4-diphenyl ether-4 pyridyl-triphenylamine-(6-bromohexyl)-triphenylphosphine (OPY-TPA-TPP), has the following Show molecular structure:
[0048]
[0049] The preparation method of above-mentioned OPY-TPA-TPP, comprises the steps:
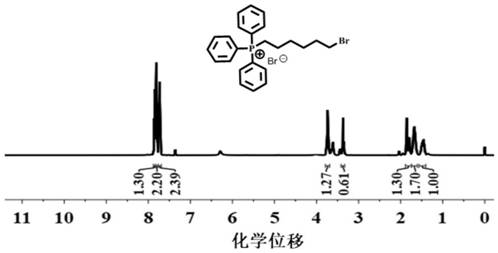
[0050] S1. Mix triphenylphosphine (10mmol, 2.62g) and 1,6-dibromohexane (20mmol, 4.86g) in tetrahydrofuran (100mL) and react at 120°C for 72 hours to obtain a white viscous product Crude product (6-bromohexyl)-triphenylphosphine (1,6-TPP); after washing 3 times with diethyl ether, the viscous product solidified and was recrystallized with three mixed solvents of tetrahydrofuran, methanol and n-hexane to obtain a white Crystalline 1,6-TPP (5mmol, 2.53g, yield 50%) (the NMR results are as figure 1 shown);
[0051] Its reaction equation is as follows:
[0052]
[0053] Mix 4,4,4-tribromotriphenylamine (5mmol, 2.41g) with 4-boronic diphenyl ether (10mmol, 2.14g), tetrakistrip...
Embodiment 2
[0063] A kind of triphenylamine-triphenylphosphine compound name is 4-4-dibromo-4 pyridyl-triphenylamine-(6-bromohexyl)-triphenylphosphine (Br-TPA-TPP), has as follows Molecular Structure:
[0064]
[0065] The preparation method of above-mentioned Br-TPA-TPP, comprises the steps:
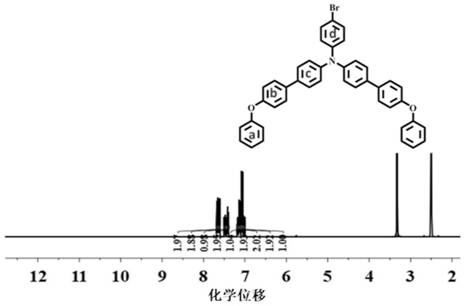
[0066] S1. Combine 4,4,4-tribromotriphenylamine (5mmol, 2.41g), 4-pyridineboronic acid (3mmol, 3.69g), tetrakistriphenylphosphopalladium (0.15mmol, 173.3mg) and potassium carbonate (3mmol, 414mg) was mixed in a mixed solution of methanol and tetrahydrofuran with a volume ratio of 1:1, and reacted at 90°C for 24h under nitrogen protection to obtain 4-4-dibromo-4-pyridyl-triphenylamine (Br-TPA-PY) crude product (The NMR results are as Figure 4 shown), purified by column chromatography to obtain a light yellow solid, the solid has a faint blue fluorescence, and the yield is 35%;
[0067] Its reaction equation is as follows:
[0068]
[0069] S2. Dissolve Br-TPA-PY (1mmol, 506mg) and 1,6-tri...
Embodiment 3
[0073] A kind of triphenylamine-triphenylphosphine compound name is 4-pyridyl-triphenylamine-(6-bromohexyl)-triphenylphosphine (TPA-TPP), has molecular structure as shown below:
[0074]
[0075] The preparation method of above-mentioned TPA-TPP, comprises the steps:
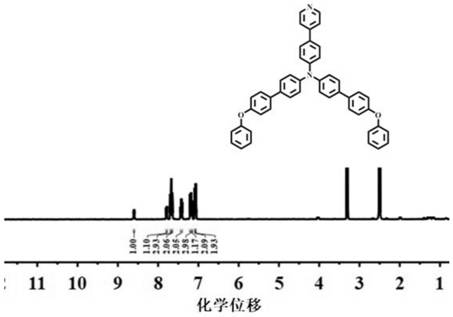
[0076] S1. Mix 4-bromotriphenylamine (10mmol, 3.23g) with 4-pyridine boronic acid (10mmol, 1.23g), dissolve tetrakistriphenylphosphopalladium (0.5mmol, 577.78mg) and potassium carbonate (10mmol, 1.38g) In a mixed solution of methanol and tetrahydrofuran with a volume ratio of 1:1, the crude product of 4-pyridyl-triphenylamine (TPA-PY) was obtained by reacting at 90° C. under nitrogen protection for 24 h (its NMR results are as follows: Figure 5 shown), purified by column chromatography to obtain a light yellow solid, the solid has blue fluorescence, and the yield is 80%;
[0077] Its reaction equation is as follows:
[0078]
[0079] S2. Dissolve TPA-PY (386mg, 1.2mmol) and 1,6-triphenylphosphine (1mmol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com