Preparation method of dopamine hydrochloride
A technology of dopamine hydrochloride and dobutamine hydrochloride, which is applied in the field of medicinal chemistry, can solve the problems of harsh reaction conditions, high cost of decarboxylase, and unsuitability for industrial production, and achieve stable properties, not easy to be oxidized and degraded, and conducive to the control of impurities Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
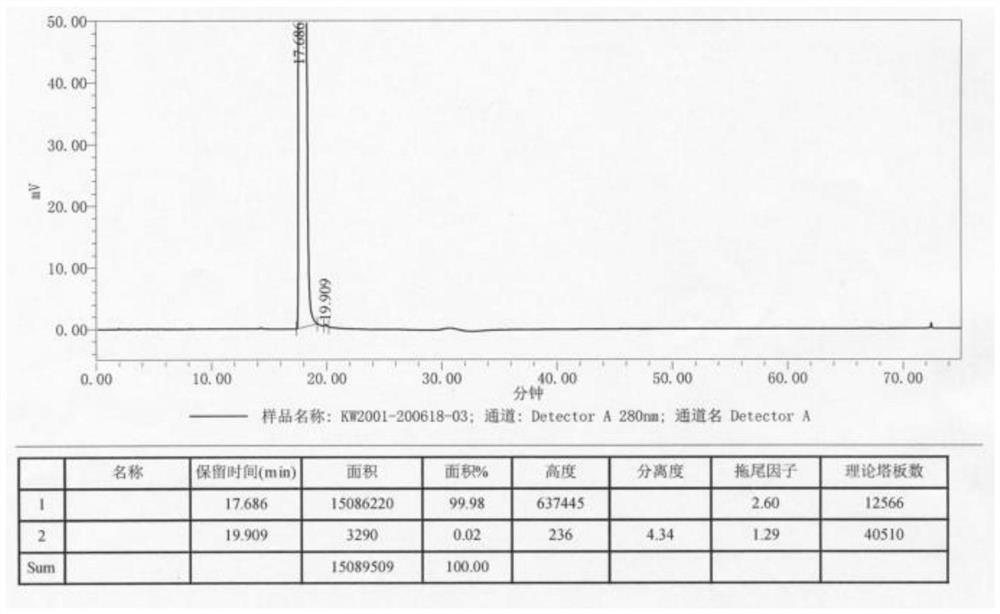
Image
Examples
Embodiment 1
[0036] A kind of preparation method of dopamine hydrochloride, concrete steps are:
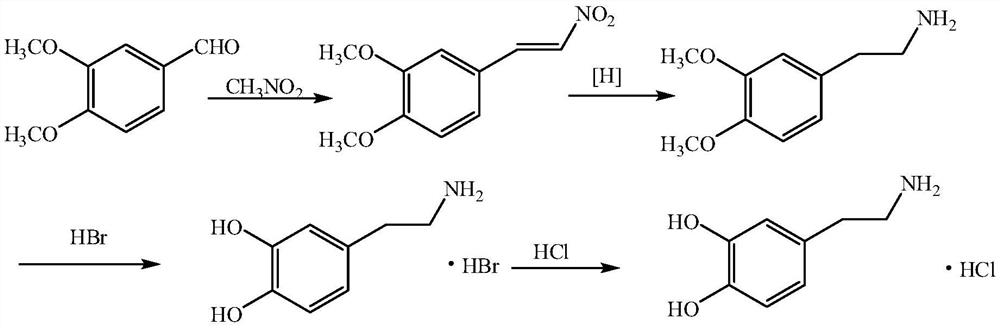
[0037] 1. Synthesis of 3,4-dimethoxy-β-nitrostyrene
[0038] Add 6.8g of methylamine hydrochloride, 166.2g of veratraldehyde, 73.3g of nitromethane and 900ml of ethanol into a 2L three-necked flask, stir and dissolve, add dropwise 11.11g of triethylamine, and condense with stirring at room temperature. During the reaction, solid Gradually precipitate out. After the reaction, filter with suction, wash the filter cake with a small amount of cold ethanol, and dry under vacuum at 40°C to obtain 193.1 g of light yellow solid 3,4-dimethoxy-β-nitrostyrene, with a yield of 92.1%.
[0039] 2. Synthesis of 3,4-dimethoxyphenethylamine
[0040] Add 190.0g of 3,4-dimethoxy-β-nitrostyrene, 5% palladium carbon (19.0g, 50% water content), 572.2g of ammonium formate, and 1350ml of methanol into a 2L three-necked flask, under nitrogen protection, and stir. Raise the temperature to 60-65°C and react for 6-8 h...
Embodiment 2
[0046] A kind of preparation method of dopamine hydrochloride, concrete steps are:
[0047] 1. Synthesis of 3,4-dimethoxy-β-nitrostyrene
[0048] Add 10g of 30% methylamine ethanol solution, 166.2g of veratraldehyde, 73.3g of nitromethane and 900ml of ethanol into a 2L three-necked flask, stir, dissolve, and condense with stirring at room temperature. During the reaction, solids gradually precipitate out. After the reaction, filter with suction, wash the filter cake with a small amount of cold ethanol, and dry under vacuum at 40°C to obtain 186.5 g of light yellow solid 3,4-dimethoxy-β-nitrostyrene with a yield of 89.1%.
[0049] 2. Synthesis of 3,4-dimethoxyphenethylamine
[0050]Add 180.0g of 3,4-dimethoxy-β-nitrostyrene, 5% palladium carbon (18.0g, 50% water content), and 900ml of methanol into a 3L three-necked flask, protect with nitrogen, stir, and control the temperature for 20-30 ℃, add 1000.0 g of triethylsilane dropwise, and keep the reaction for 4 to 5 hours after...
Embodiment 3
[0056] A kind of preparation method of dopamine hydrochloride, concrete steps are:
[0057] 1. Synthesis of 3,4-dimethoxy-β-nitrostyrene
[0058] Add 6.8g of methylamine hydrochloride, 166.2g of veratraldehyde, 73.3g of nitromethane and 900ml of ethanol into a 2L three-necked flask, stir and dissolve, add 8.03g of diethylamine dropwise, and condense with stirring at room temperature. During the reaction, solid Gradually precipitate out. After the reaction, filter with suction, wash the filter cake with a small amount of cold ethanol, and dry under vacuum at 40°C to obtain 187.0 g of light yellow solid 3,4-dimethoxy-β-nitrostyrene, with a yield of 89.4%.
[0059] 2. Synthesis of 3,4-dimethoxyphenethylamine
[0060] Add 187.0g of 3,4-dimethoxy-β-nitrostyrene, 8.0g of 10% palladium carbon, 400g of hydrazine hydrate, and 900ml of methanol into a 3L three-necked flask, protect with nitrogen, stir, and heat up to 60-65°C for reaction 6~8h. After the reaction was complete, the pH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com