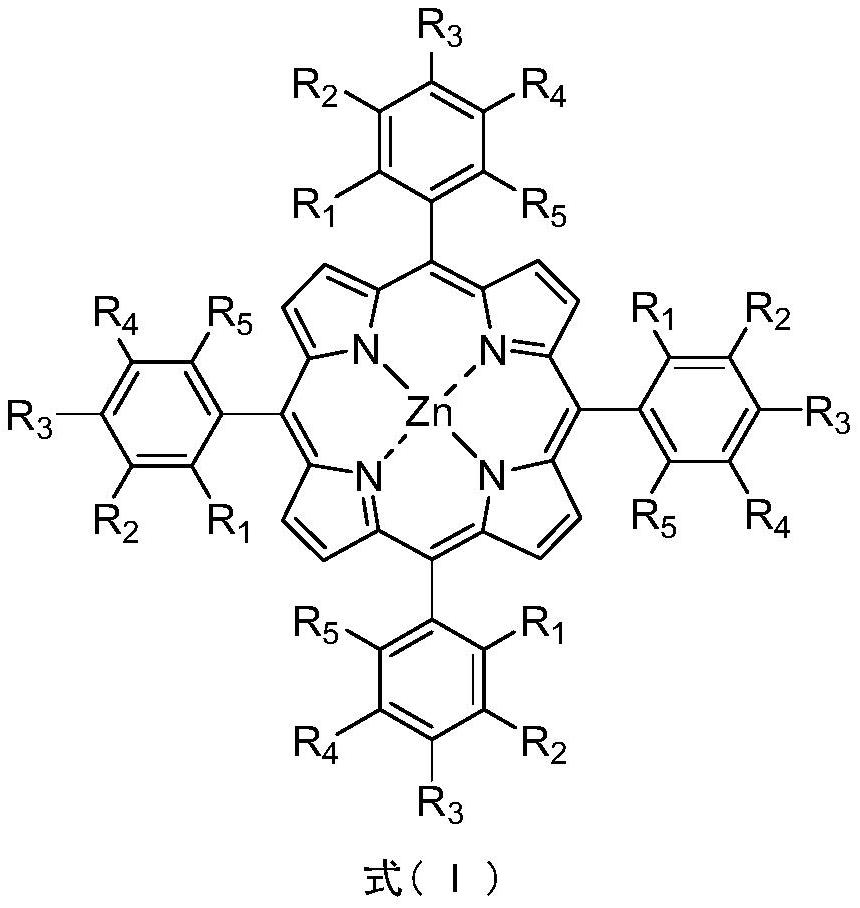
Method for synthesizing cumene hydroperoxide through catalytic oxidation of cumene by zinc (II) porphyrin
A porphyrin-catalyzed technology for oxidizing cumene and cumene hydrogen peroxide, which can be used in the preparation of peroxy compounds, chemical instruments and methods, preparation of organic compounds, etc., and can solve the problem that the selectivity of cumene hydrogen peroxide is not high enough , The conversion rate of cumene is not ideal, the safety factor is low, etc., to achieve the effect of improving atomic economy, small environmental impact, and reducing emissions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 52
[0023] Embodiment 52 is a scale-up experiment.
Embodiment 1
[0025] In a 25mL reaction tube, disperse 0.0009g (0.001mmol) 5,10,15,20-tetrakis(4-carboxyphenyl)porphyrin zinc (II) in 1.2019g (10mmol) cumene, stir and heat up to 80°C, feed oxygen (0.20MPa). At 80°C, the reaction was stirred at 800rpm for 8.0h. After completion of the reaction, cool to room temperature, add 1.3115g (5.00mmol) triphenylphosphine (PPh 3 ), stirred at room temperature for 40min to reduce the generated peroxide. Using acetone as solvent, the resulting reaction mixture was adjusted to 50 mL. Pipette 10 mL of the resulting solution, and use naphthalene as an internal standard for gas chromatographic analysis. The conversion rate of cumene was 16.5%, the selectivity of 2-phenyl-2-propanol was 0.9%, the selectivity of acetophenone was 0%, the selectivity of cumene hydroperoxide was 99.1%, and the formation of benzoic acid was not detected.
Embodiment 2
[0027] In a 25mL reaction tube, disperse 0.0009g (0.001mmol) 5,10,15,20-tetrakis(3-carboxyphenyl)porphyrin zinc (II) in 1.2019g (10mmol) cumene, stir and heat up to 80°C, feed oxygen (0.20MPa). At 80°C, the reaction was stirred at 800rpm for 8.0h. After completion of the reaction, cool to room temperature, add 1.3115g (5.00mmol) triphenylphosphine (PPh 3 ), stirred at room temperature for 40min to reduce the generated peroxide. Using acetone as solvent, the resulting reaction mixture was adjusted to 50 mL. Pipette 10 mL of the resulting solution, and use naphthalene as an internal standard for gas chromatographic analysis. The conversion rate of cumene was 9.1%, the selectivity of 2-phenyl-2-propanol was 0.0%, the selectivity of acetophenone was 0.0%, the selectivity of cumene hydroperoxide was 100%, and the formation of benzoic acid was not detected.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com