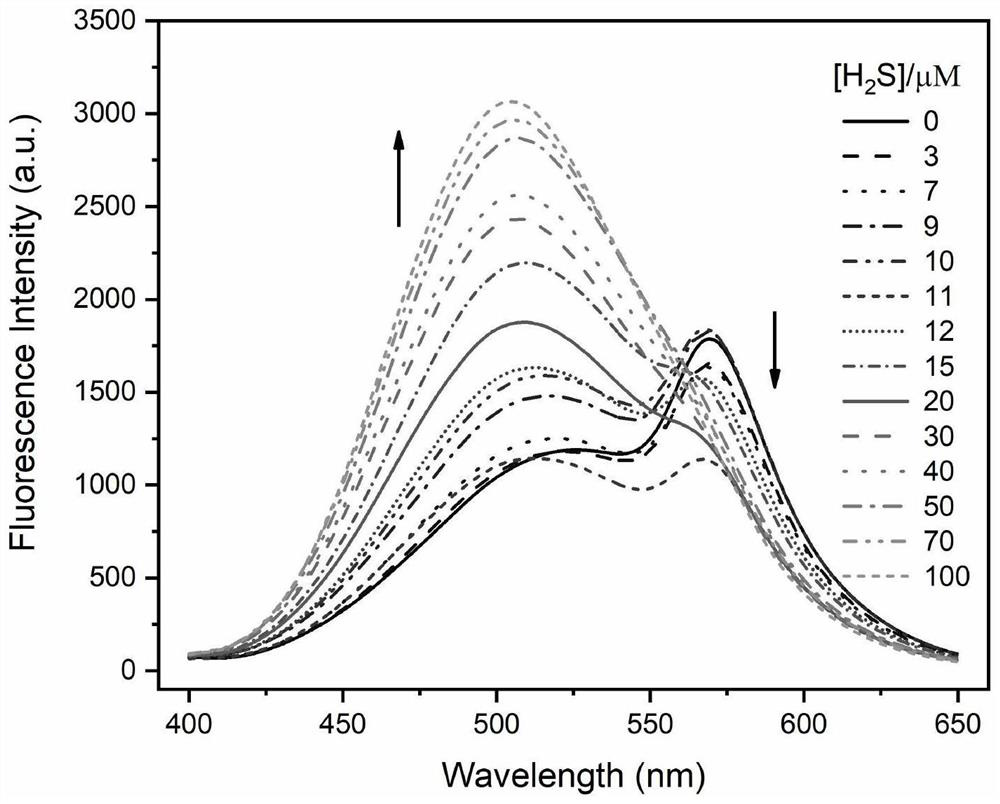
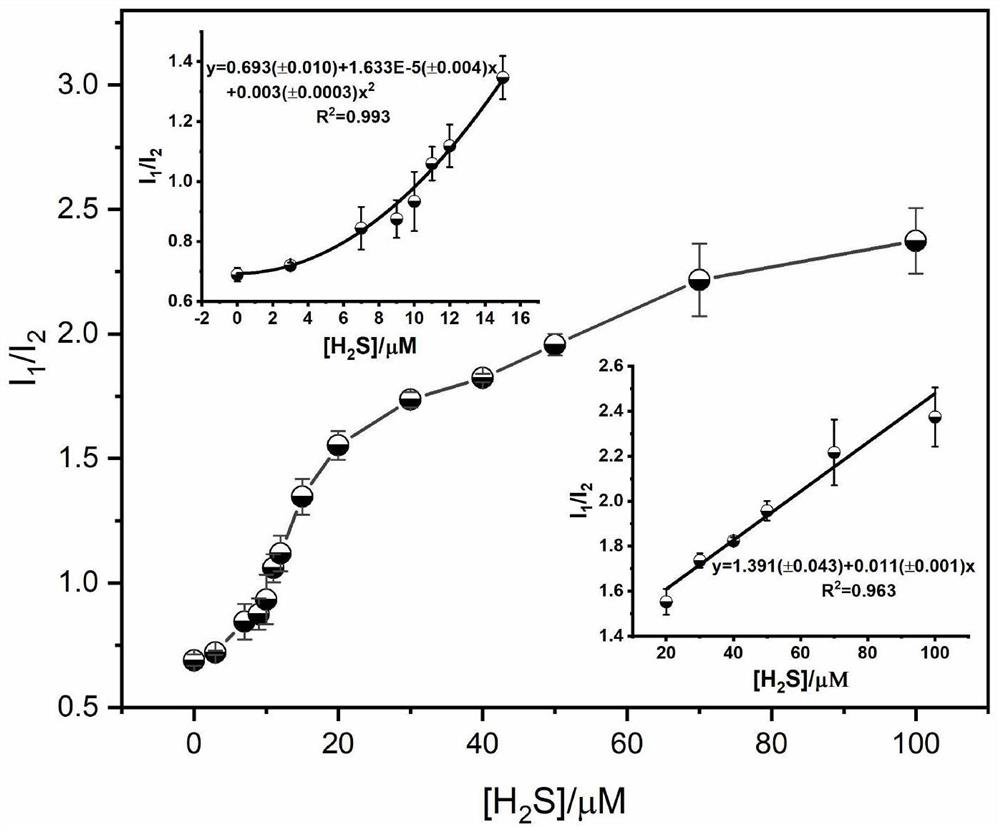
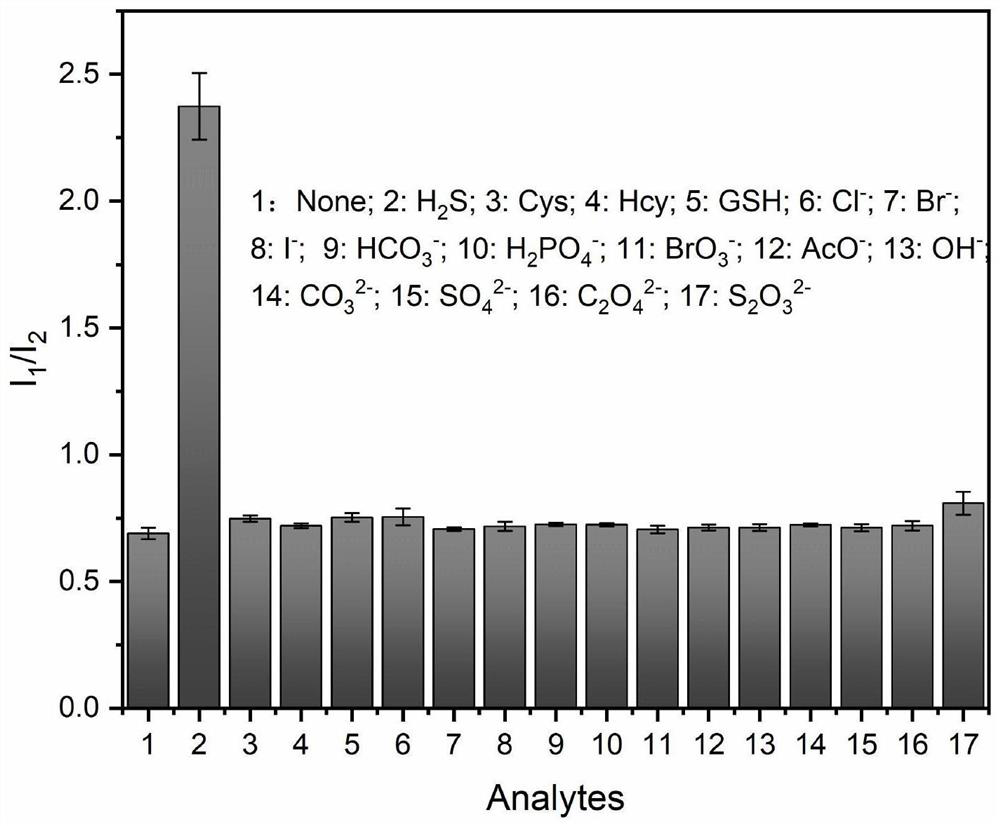
Dansyl derivative fluorescent probe as well as synthesis method and application thereof
A technology of dansyl derivatives and fluorescent probes, applied in the field of dansyl derivative fluorescent probes and their synthesis, can solve the problems of complex operation, low selectivity, low sensitivity and the like, and achieves good selectivity and simple synthesis , prepare a simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Synthesize the dansyl derivative fluorescent probe with the following structural formula:
[0042]
[0043] Its synthetic steps are as follows:
[0044] Under nitrogen protection at a flow rate of 0.6mL / s and at room temperature, a chloroform solution containing 0.50 g (1.85 mmol) of dansyl chloride was added dropwise to a solution containing 253 μL (2.78 mmol) of 4-bromo-1-butanol and 385 μL of (2.78mmol) triethylamine in chloroform solution, after the dropwise addition was completed, the reaction was stirred at room temperature for 4 hours. Washed with saturated brine for 6 times, then dried with anhydrous sodium sulfate for 4 h, distilled off chloroform, and purified the product by column chromatography using a mixed solvent of dichloromethane and petroleum ether with a volume ratio of 1.5:1 as eluent, Obtain dansyl derivative fluorescent probe, its yield is 86%, and its reaction equation is as follows:
[0045]
[0046] The NMR data of the above-mentioned da...
Embodiment 2
[0048] Synthesize the dansyl derivative fluorescent probe with the following structural formula:
[0049]
[0050] Its synthetic steps are as follows:
[0051] In the synthesis steps of Example 1, the 4-bromo-1-butanol used was replaced by 6-bromo-1-hexanol with an equimolar mass, and other steps were the same as the corresponding Example 1.
Embodiment 3
[0053] Synthesize the dansyl derivative fluorescent probe with the following structural formula:
[0054]
[0055] Its synthetic steps are as follows:
[0056] In the synthesis step of Example 1, the 4-bromo-1-butanol used was replaced with 8-bromo-1-octanol of equimolar mass, and other steps were the same as the corresponding Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com