Novel coronavirus SARS-CoV-2 safe replication subsystem and application thereof
A coronavirus, sars-cov-2 technology, applied in the direction of viruses, applications, viral peptides, etc., can solve problems such as time-consuming, labor-intensive, large molecular weight, and lack of expression level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] Construction of Example 1 Replicon
[0099] Based on the genome composition of the new coronavirus and the principle process of viral RNA synthesis (replication and transcription process), the inventor's team creatively constructed a safe replicon of the new coronavirus SARS-CoV-2, including the following two expression structures :
[0100] (I) encoding the non-structural protein of the novel coronavirus SARS-CoV-2;
[0101] (II) The 5'UTR and 3'UTR of the new coronavirus SARS-CoV-2, and the transcriptional regulatory regions and reporter genes that the non-structural proteins of the new coronavirus SARS-CoV-2 can act on.
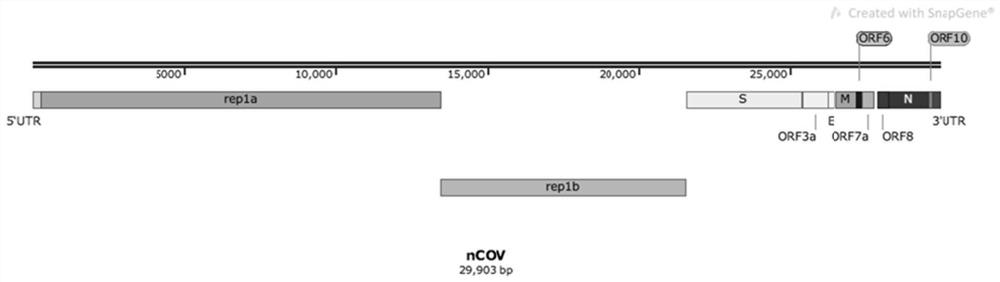
[0102] (I) The non-structural protein encoding the novel coronavirus SARS-CoV-2 is an expression vector encoding the nsp1-nsp16 protein sequence.
[0103] The sequences of rep1a and rep1b in the genome of the new coronavirus total about 20,000 bp, accounting for about 2 / 3 of the virus genome. Considering the efficiency of transfection and express...
Embodiment 2
[0174] The establishment of embodiment 2 novel coronavirus SARS-CoV-2 replicon system
[0175] The purpose of constructing the replicon system in Example 1 is to screen anti-new coronavirus SARS-CoV-2 drugs, especially human drugs, so the HEK 293T cell line was selected as packaging cells for verification. The schematic diagram of the working principle of ps2V, ps2AN, ps2AC, ps2B4 expression vectors in human body or human cells is attached Image 6 shown.
[0176] HEK293T cells in good growth state were evenly spread in 12-well culture plates treated with poly-lysine (cell density was about 6.5×10 4 / cm 2 ), the cells are required to be single and evenly distributed. After about 24 hours of culture, the cell confluency should be close to 80%. At this point, the Opti-Lipo2000-DNA mixture was prepared as shown in Table 1 for transfection.
[0177] The concentration of the four carriers can be in the range of 0.01-1 μg / μL, and the ratio of the four carriers can be adjusted w...
Embodiment 3
[0182] Embodiment 3 detects the performance of the new coronavirus SARS-CoV-2 replicon system
[0183] The ps2V, ps2AN, ps2AC, and ps2B plasmids were transfected according to the steps in Example 2. 6h after transfection, Remdesivir (Remdesivir), lopinavir ( Lopinavir), Ritonavir (Ritonavir). After 24 hours of drug treatment, the cell luciferase activity was detected, and the inhibition rate was calculated based on the DMSO control, and the half-inhibitory concentration of the drug (hereinafter referred to as IC50) was calculated using GraphpadPrism 7.0 software. For specific results, see Figures 10 to 12 .
[0184] in Figure 10 The results showed that the IC50 of Remdesivir was 12.4±1.08μM; Figure 11 The results showed that the IC50 of Lopinavir was 6.785±1.09μM; Figure 12 The results showed that the IC50 of Ritonavir was 14.77±1.05 μM.
[0185] The above data results show that the replicon system constructed in Example 1 can reproduce the response of wild-type SAR...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com