Ophthalmic preparation and preparation method and application thereof
An ophthalmic preparation and content technology, applied in the field of protein engineering, can solve the problem of unsatisfactory transthyretin effect and achieve the effect of improving the therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Embodiment 1 Recombinant preparation of human transthyretin (TTR)
[0067] (1) Construction of the recombinant plasmid pETx-rhaPBAD-ttr: transform and restructure the pET-21a plasmid (purchased from the ATCC China Culture Collection Center) (the difference in sequence between the restructured plasmid and the original pET-21a plasmid is About 75%, the specific sequence is shown in SEQ ID NO: 4), and the rhaPBAD promoter (Rhamna Tang inducible) is used to replace the T7 promoter, and the human TTR optimized nucleic acid sequence is connected at the same time (as shown in SEQ ID NO: 2 , the amino acid sequence of TTR is shown in SEQ ID NO:1), and the overall nucleic acid sequence of the resulting plasmid is shown in SEQ ID NO:3. After sequencing verification (the sequencing company is Nanjing GenScript Biotechnology Co., Ltd.), the construction was successful.
[0068] (2) Expression and purification of recombinant human TTR: transform the pETx-rhaPBAD-ttr plasmid constru...
Embodiment 2
[0071] Example 2 Computer Simulation of the Binding Form of TTR and Each Ligand Molecule
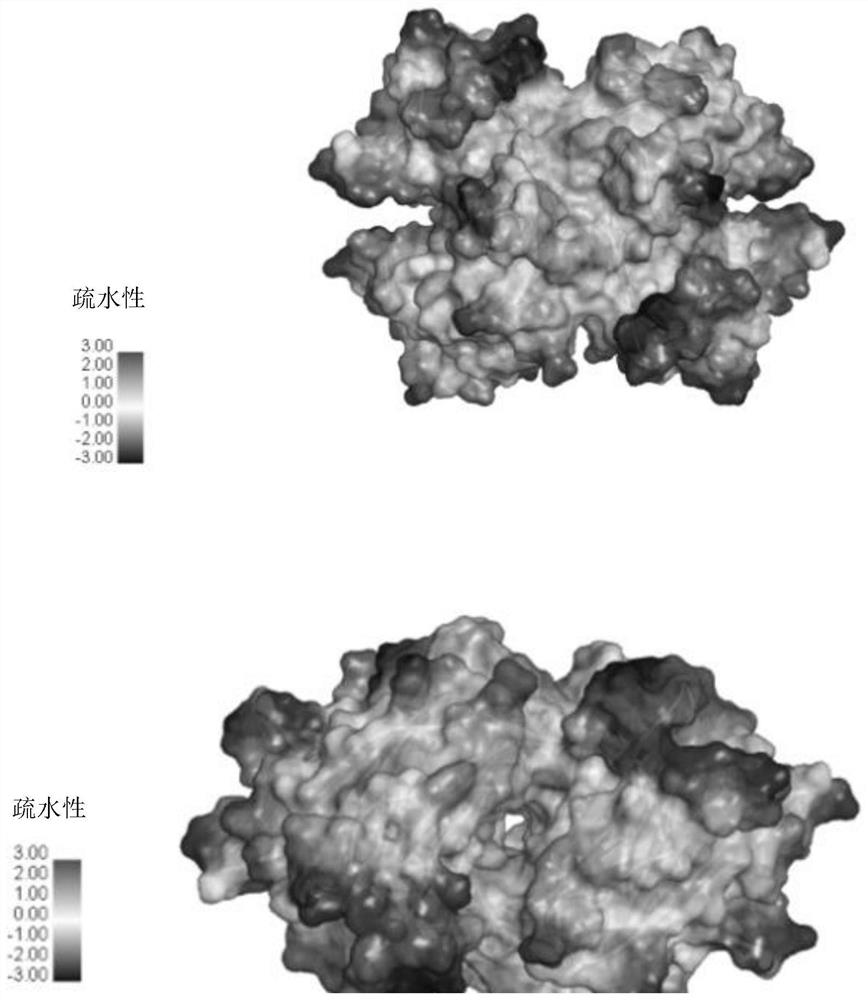
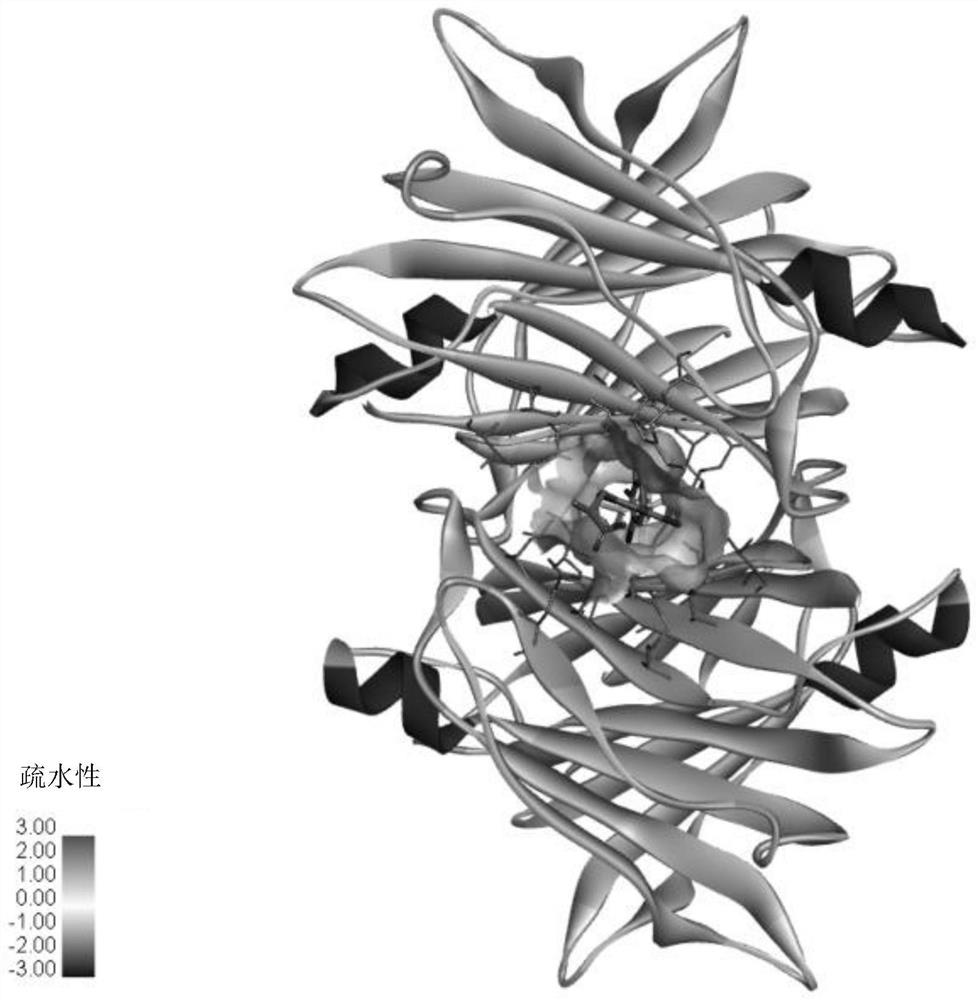
[0072] Refer to the static co-crystal model (PDF: 3CFQ) of TTR dimer and diclofenac in the PDB database, such as Figure 4A shown. Figure 4AIn , the protein structure is a TTR dimer, and the diclofenac ligand molecule is indicated by an arrow, and one molecule of TTR dimer can bind two molecules of diclofenac. Figure 4B The interaction of diclofenac with TTR amino acid residues is shown in .
[0073] Molecular simulation by Discovery studio software found that vitamin A1 (retinol) can stably bind to the hydrophobic channel of TTR polymer. Figure 5A The protein structure shown in is a TTR dimer, the vitamin A1 ligand molecule is indicated by an arrow, and one molecule of TTR dimer can bind one molecule of vitamin A1. Figure 5B The interaction of vitamin A1 with TTR amino acid residues is shown in .
[0074] Molecular simulation by Discovery studio software found that vitamin A2 (3...
Embodiment 3
[0077] Example 3 Determination of dynamic specific binding parameters between TTR and each potential ligand molecule
[0078] Use nano ITC (TA) to measure the affinity binding equilibrium dissociation constant K of TTR and each ligand molecule mentioned in Example 2 or its salt d , in 10 μmol / L TTR solution (1000 μL), dropwise add 100 μmol / L of the above-mentioned various ligand solutions at a rate of 1 μL / min, and use the built-in software to calculate the affinity binding equilibrium dissociation constant K d (Table 2).
[0079] Table 2 The affinity binding equilibrium dissociation constants of various ligand molecules and TTR
[0080]
[0081] As shown in Table 2, the equilibrium dissociation constants of affinity binding between various ligand molecules and TTR are close to 10 -8 mol / L, which is close to the ability of monoclonal antibodies to recognize a single epitope, indicating that the above-mentioned ligand molecules can specifically recognize and bind to TTR. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com