A kind of ab-type monomer based on piperidine alkyne-azido group and its preparation method and application
A piperidinyl methyl azide, AB-type technology is applied in the field of alkyne-azide-based AB-type monomer and its preparation, which can solve the problems of ion conductivity and mechanical stability, and poor mechanical stability. Good hydroxide ion conductivity and battery performance, inhibition of swelling due to water absorption, and the effect of improving ion conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The present invention provides the preparation method for preparing polyphenylene ether comb-shaped long side chain polymer described in the above technical scheme, comprising the following steps:
[0034] Under a protective atmosphere, click reaction is carried out between the above-mentioned type AB monomer and azide polyphenylene ether to be modified to obtain an anion exchange membrane containing comb-like long side chains.
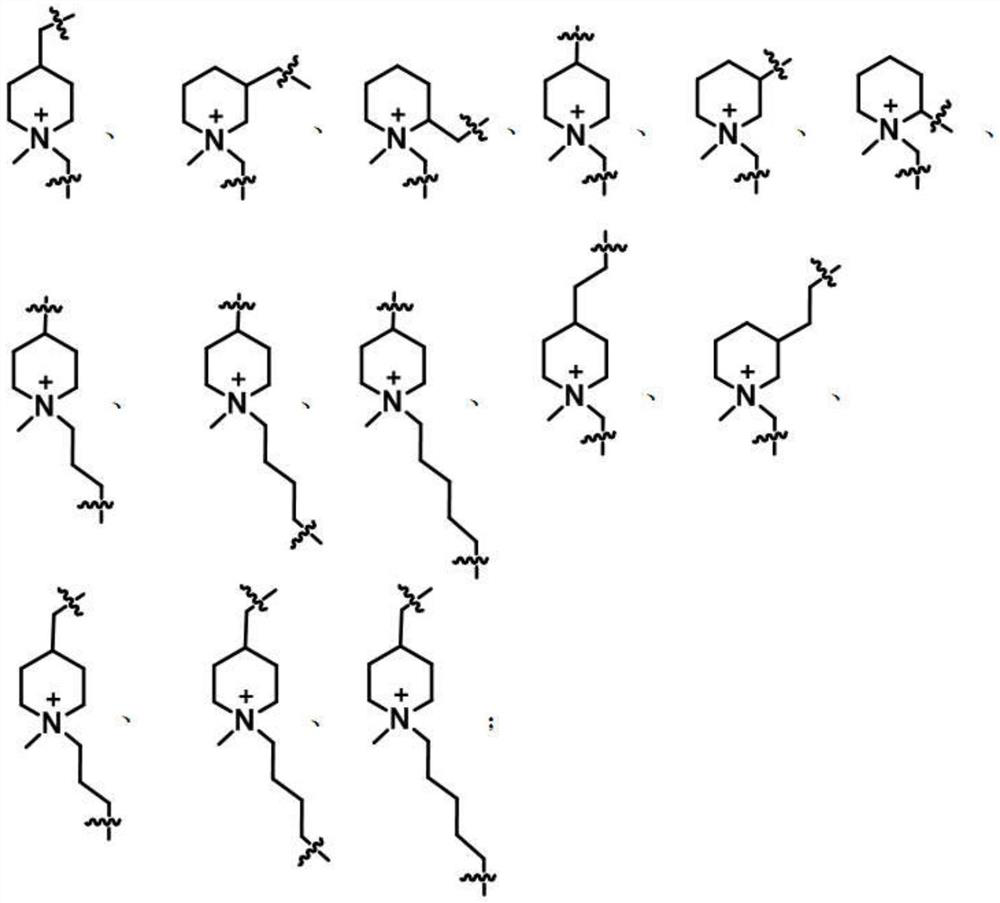
[0035]The AB type monomers include N-methyl-propynyl-4-piperidinyl azide, N-methyl-propynyl-3-piperidinyl azide, N-methyl-propynyl Alkynyl-2-piperidinyl azide, N-methyl-propynyl-4-piperidinyl azide, N-methyl-propynyl-3-piperidinyl azide, N-methyl-propynyl Alkynyl-2-piperidine azide, N-methyl-butynyl-4-piperidine azide, N-methyl-pentynyl-4-piperidine azide, N-methyl-hexynyl -4-piperidine azide, N-methyl-propynyl-4-piperidine ethyl azide, N-methyl-propynyl-3-piperidine ethyl azide, N-methyl-butyl A kind of alkynyl-4-piperidylmethyl azide, N-met...
Embodiment 1
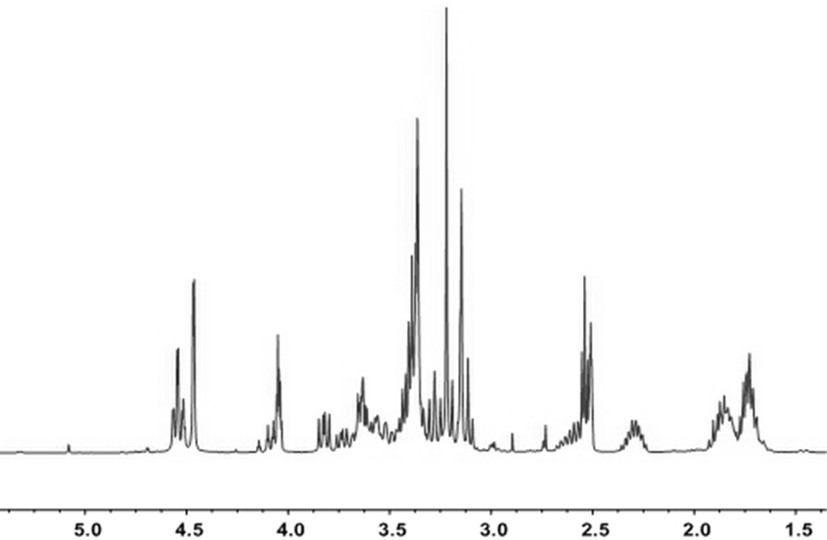
[0058] Synthesis of type AB monomers:
[0059] In a completely dry 150ml single-necked bottle, under nitrogen protection, N-methyl-4-piperidinemethanol (2.58g, 20mmol) was dissolved in 50ml of dichloromethane, and p-toluenesulfonyl chloride (5.88g, 30mmol) and pyridine (2.34g, 30mmol), at room temperature, magnetic stirring to make it completely dissolved, after reacting for 24h, pour it into 30ml saturated NaHCO 3 The reaction is carried out in the solution, the reaction time is 10h, and then the solution is layered, and anhydrous magnesium sulfate is added to the obtained lower layer solution for dehydration treatment, and then dichloromethane is suspended and dried to obtain a crude product, which is washed with ether 3 times, drying at room temperature to obtain the sulfonylated crude product, yield: 70%. The reaction formula is as follows:
[0060]
[0061] In a completely dry 150ml single-necked bottle, under nitrogen protection, add the sulfonylated crude product (...
Embodiment 2
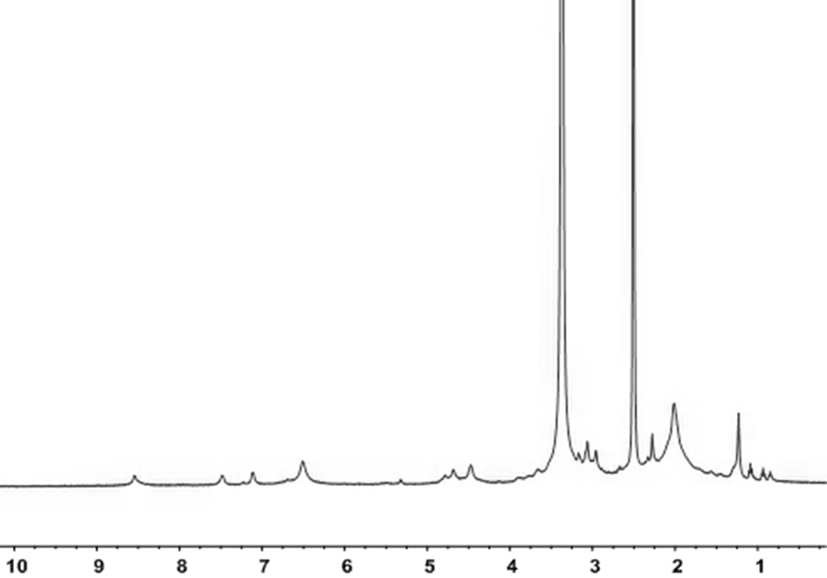
[0066] Synthesis of azide polyphenylene ether:
[0067] In a completely dry 150ml single-necked bottle, polyphenylene ether (1.2g, 10mmol) was successively added under nitrogen protection, and it was dissolved in 60ml of chlorobenzene, and the temperature of the polymer was slowly raised to 120°C and bromobutylene was added to Imide (0.83g, 4.68mmol) and azobisisobutyronitrile (0.06g, 0.36mmol), then the temperature was slowly raised to 135°C for 5h, and the reaction solution was precipitated in 500ml of methanol 15% brominated polyphenylene ether was obtained by nuclear magnetic analysis, and washed three times with methanol to obtain brominated polyphenylene ether, the product was 90%, and the reaction formula was as follows:
[0068]
[0069] In a completely dry 150ml single-necked bottle, brominated polyphenylene ether (1.42g, 10mmol) was added successively under nitrogen protection, it was dissolved in 60ml of NMP, and sodium azide (3.25g, 50mmol) was added thereto Th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com