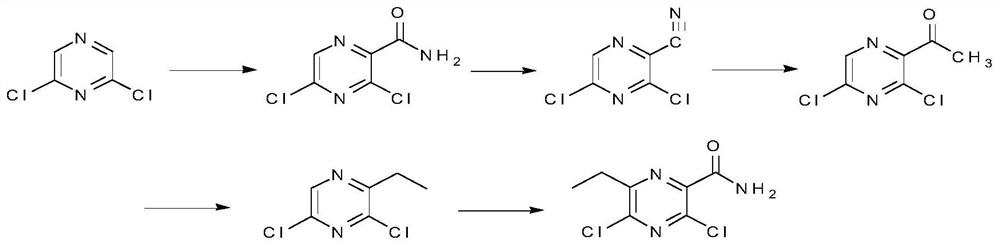
Synthesis method of 3,5-dichloro-6-ethylpyrazineformamide
A technology of ethylpyrazinecarboxamide and dichloropyrazinecarboxamide is applied in the synthesis field of 3,5-dichloro-6-ethylpyrazinecarboxamide, and can solve the problems of high price and not clearly indicated, etc., To achieve the effect of short synthesis process, mild step operation and reaction conditions, easy control and industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: a kind of synthetic method of 3,5-dichloro-6-ethylpyrazine carboxamide comprises the following steps:
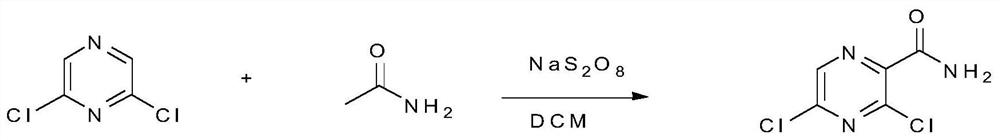
[0039] Step 1: Heat 330g of 2,6-dichloropyrazine and 1980g of formamide to 90°C, add 513g of sodium persulfate in three batches; react for 10 minutes after the addition, add 2500ml of water; cool to room temperature (ie 25°C) After each 1000ml of methylene chloride was extracted 4 times, the organic phases were combined, and the combined organic phase was washed three times with 150 ml of saturated brine, followed by drying with anhydrous sodium sulfate to remove the solvent to obtain a off-white solid, i.e. 3, 281 g of 5-dichloropyrazine carboxamide, yield 65.9%.
[0040] 3,5-Dichloropyrazine carboxamide NMR data:
[0041] CDCl 3 ,CONH(6.342,br),CONH(7.415,br),CH(8.491,s).
[0042] Step 2: Add 150 g of 3,5-dichloropyrazine carboxamide obtained in Step 1 into 200 g of phosphorus oxychloride, and heat to reflux for 4 hours. Cool and pour into 1L of ic...
Embodiment 2
[0050] Embodiment 2: a kind of synthetic method of 3,5-dichloro-6-ethylpyrazine carboxamide comprises the following steps:
[0051] Step 1: Heat 330g of 2,6-dichloropyrazine and 1950g of formamide to 95°C, add 428.6g of sodium persulfate in four batches; react for 12 minutes after the addition, add 2000ml of water; cool to room temperature (20°C) Each 1000ml of dichloromethane was extracted 4 times, the organic phases were combined, and the combined organic phase was washed three times with 200ml of saturated brine, followed by drying with anhydrous sodium sulfate to remove the solvent to obtain a off-white solid, i.e. 3,5 -Dichloropyrazine carboxamide 295g, the yield is 69.3%.
[0052] Step 2: Add 150 g of 3,5-dichloropyrazine carboxamide obtained in Step 1 to 179.3 g of phosphorus oxychloride, and heat to reflux for 4.5 hours. Cool and pour into 1L of ice water, add 500ml of dichloromethane each time for extraction, combine the organic phases, and wash the combined organic ...
Embodiment 3
[0056] Embodiment 3: a kind of synthetic method of 3,5-dichloro-6-ethylpyrazine carboxamide comprises the following steps:
[0057] Step 1: Heat 330g of 2,6-dichloropyrazine and 2340g of formamide to 100°C, add 523.8g of sodium persulfate in three batches; react for 15 minutes after the addition, add 3000ml of water; cool to room temperature (25°C) Each 1000ml of dichloromethane was extracted 4 times, the organic phases were combined, and the combined organic phase was washed three times with 250 ml of saturated brine, followed by drying with anhydrous sodium sulfate to remove the solvent to obtain a off-white solid, i.e. 3,5 -Dichloropyrazine carboxamide 310g, the yield is 72.9%.
[0058] Step 2: Add 150 g of 3,5-dichloropyrazine carboxamide obtained in Step 1 to 215.2 g of phosphorus oxychloride, and heat to reflux for 5 hours. Cool and pour into 1L of ice water, add 500ml of dichloromethane each time for extraction twice, combine the organic phases, and wash the combined o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com