Method and system for supercritical fluid extraction of metal
A supercritical fluid, metal technology, applied in the process of supercritical conditions, solid solvent extraction, preparation/processing of rare earth metal compounds, etc., can solve problems such as difficulty in dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
[0087] Preferred Embodiment 1: Extraction of Rare Earth Elements from Hybrid Electric Vehicle Batteries
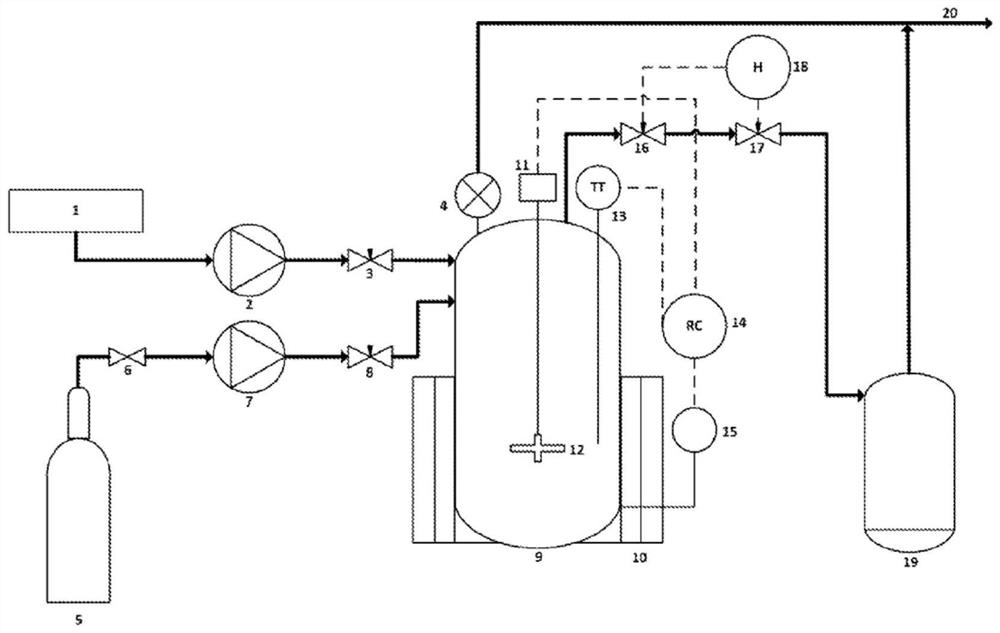
[0088] The present invention relates to the development of an efficient and sustainable process for the urban mining of rare earth elements from waste electrical and electronic equipment, such as nickel metal hydride batteries. In a preferred embodiment, the developed process relies on the utilization of CO 2 Supercritical fluid extraction as a solvent that is inert, safe and abundant. The process is efficient in the sense that it operates safely, at low temperatures, produces no hazardous waste, and preferably recovers up to about 90% of the rare earth elements.
[0089] Furthermore, the present invention provides a mechanism for supercritical fluid extraction of rare earth elements in which the trivalent rare earth element state bonded to three tri-n-butyl phosphate (TBP) molecules and three nitrates is considered for the extracted Model of the rare earth tri-n-butyl...
Embodiment approach 2
[0148] Preferred embodiment 2: Extraction of rare earth elements from permanent magnets
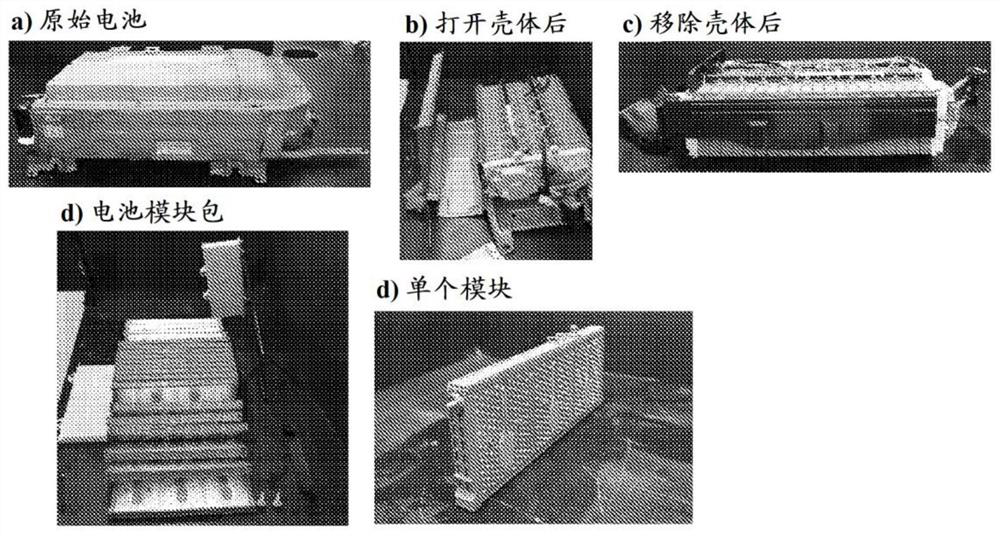
[0149] As mentioned above, the present invention relates to efficient and sustainable development for urban mining of REE from WEEE. Besides NiMH batteries, another potential source of REEs are permanent magnets that can be found in, for example, computer hard drives and wind turbines. Accordingly, another preferred embodiment of the present invention involves supercritical fluid extraction of metals, such as rare earth elements, from permanent magnets.
[0150] Experimental part of preferred embodiment 2
[0151] Chemicals and materials. The following reagents were used: Tri-n-butyl phosphate (TBP, ≥98% – from VWR TM ), concentrated nitric acid (15.7M, 70wt%–VWR TM ), concentrated hydrochloric acid (12.2M, 37wt%–VWR TM ), concentrated sodium hydroxide (19.4M, 50wt%–VWR TM ), phenolphthalein indicator solution (1% alcohol solution – VWR TM ), neodymium oxide (Nd 2 o 3 , 99.9 wt%...
Embodiment approach 3
[0216] Preferred Embodiment 3: Extraction of Rare Earth Elements from Phosphors
[0217] In yet another preferred embodiment, the source is a phosphor and the target metal is one or more rare earth elements.
[0218] Experimental part of preferred embodiment 3
[0219] The feasibility of supercritical fluid extraction of rare earth elements (strontium (Sr) and antimony (Sb)) was evaluated in a series of extraction experiments. The feedstock used for these experiments was pre-separated fluorescent bulb (FL) luminescent material from a fluorescent lamp recycling facility.
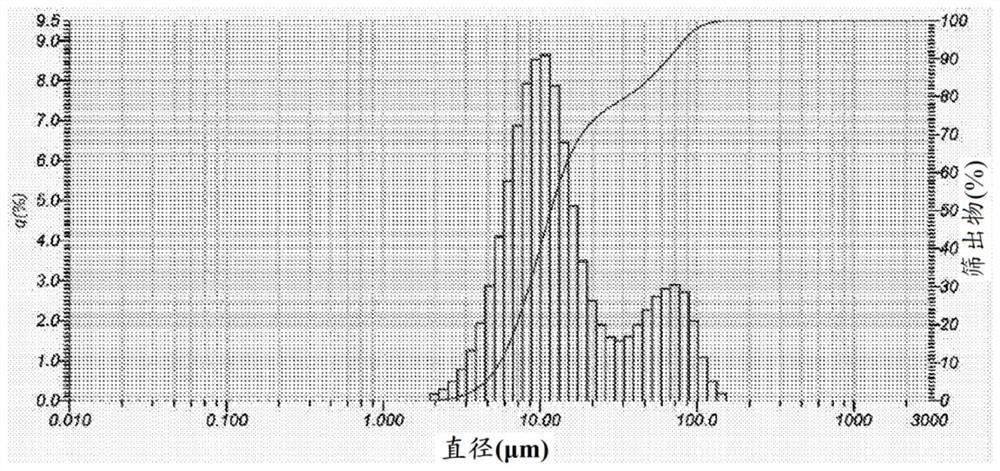
[0220] Characterization of FL luminescent materials. The physical and chemical properties of the starting luminescent materials were characterized prior to extraction experiments. The starting material is a powder corresponding to the inner coating of scrap FL. Particle size distribution determined by laser particle size analysis, such as Figure 19 shown in . A trimodal distribution corresponding to t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com