Continuous synthesis process and device for 3-bromopyridine
A technology for chemical synthesis and bromopyridine, which is applied in the field of 3-bromopyridine continuous synthesis process and equipment, can solve the problems of complex post-treatment, low reaction efficiency, and long intermittent reaction time of 3-bromopyridine, and achieve simplified reaction post-processing , Promote complete liquid separation, shorten the effect of neutralization liquid separation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
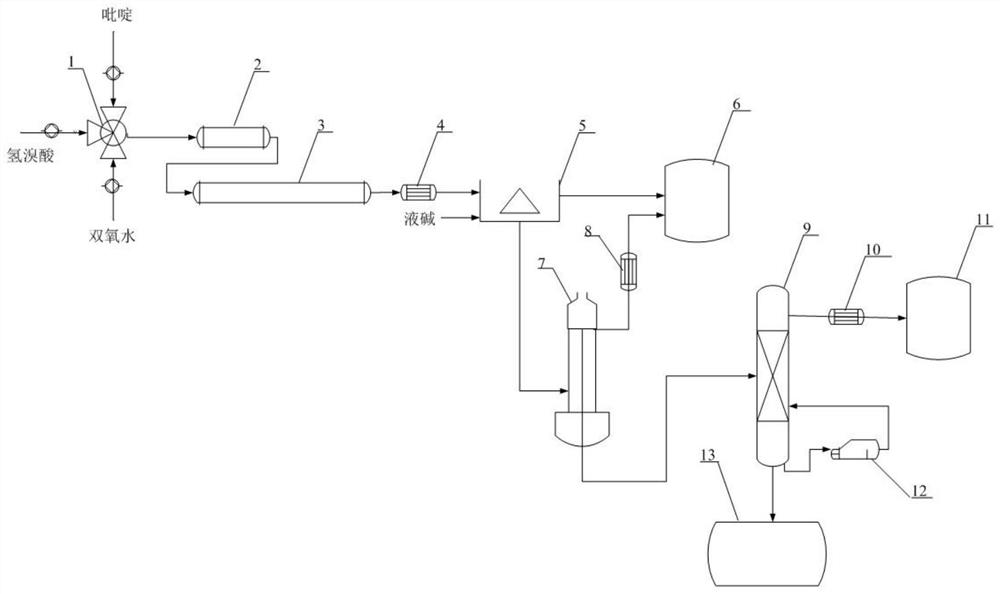
[0030] Such as figure 1 , a 3-bromopyridine continuous synthesis device, including a mixer 1, a first-stage tubular reactor 2, a second-stage tubular reactor 3, a first condenser 4, a continuous neutralization and liquid separation device 5, and a waste water storage tank 6 , negative pressure film evaporator 7, second condenser 8, negative pressure rectification tower 9, third condenser 10, product storage tank 11, reboiler 12, rectification bottom material storage tank 13, pyridine, hydrobromic acid After being metered by the metering pump, hydrogen peroxide and hydrogen peroxide are respectively connected to the three liquid inlets of the mixer 1, the liquid outlet of the mixer 1 is connected to the liquid inlet of the first-stage tubular reactor 2, and the liquid outlet of the first-stage tubular reactor 2 is connected to the second-stage pipe Type reactor 3 liquid inlet, the first-stage tubular reactor 2 is heated by steam condensed water, the second-stage tubular reactor...
Embodiment 2
[0034] The steps of 3-bromopyridine continuous synthesis technique are as follows:
[0035] Pyridine, hydrobromic acid and hydrogen peroxide are injected into the mixer 1 respectively in a molar ratio (1:4:1), the concentration of hydrobromic acid is 48%, and the concentration of hydrogen peroxide is 50%. After being mixed in the mixer 1, it enters the first-stage tubular reactor 2 for reaction. , the reaction temperature is 50°C, the residence time is 5min, the reaction liquid enters the second-stage tubular reactor 3 to continue the reaction, the reaction temperature is 90°C, the residence time is 20min, the reacted reaction liquid enters the first condenser 4 and is cooled to 10°C, after cooling The reaction liquid enters the continuous neutralization and liquid separation device 5, and 30% liquid caustic soda is added simultaneously and enters the continuous neutralization liquid separation device 5, the upper layer water phase enters the waste water storage tank 6, and the...
Embodiment 3
[0037] The steps of 3-bromopyridine continuous synthesis technique are as follows:
[0038] Pyridine, hydrobromic acid and hydrogen peroxide are injected into the mixer 1 respectively in a molar ratio (1:4:1.2), the concentration of hydrobromic acid is 49%, and the concentration of hydrogen peroxide is 50%. After being mixed in the mixer 1, it enters the first-stage tubular reactor 2 for reaction , the reaction temperature is 50°C, the residence time is 5min, the reaction liquid enters the second-stage tubular reactor 3 to continue the reaction, the reaction temperature is 90°C, the residence time is 20min, the reacted reaction liquid enters the first condenser 4 and is cooled to 10°C, after cooling The reaction liquid enters the continuous neutralization and liquid separation device 5, and 30% liquid caustic soda is added simultaneously and enters the continuous neutralization liquid separation device 5, the upper layer water phase enters the waste water storage tank 6, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com