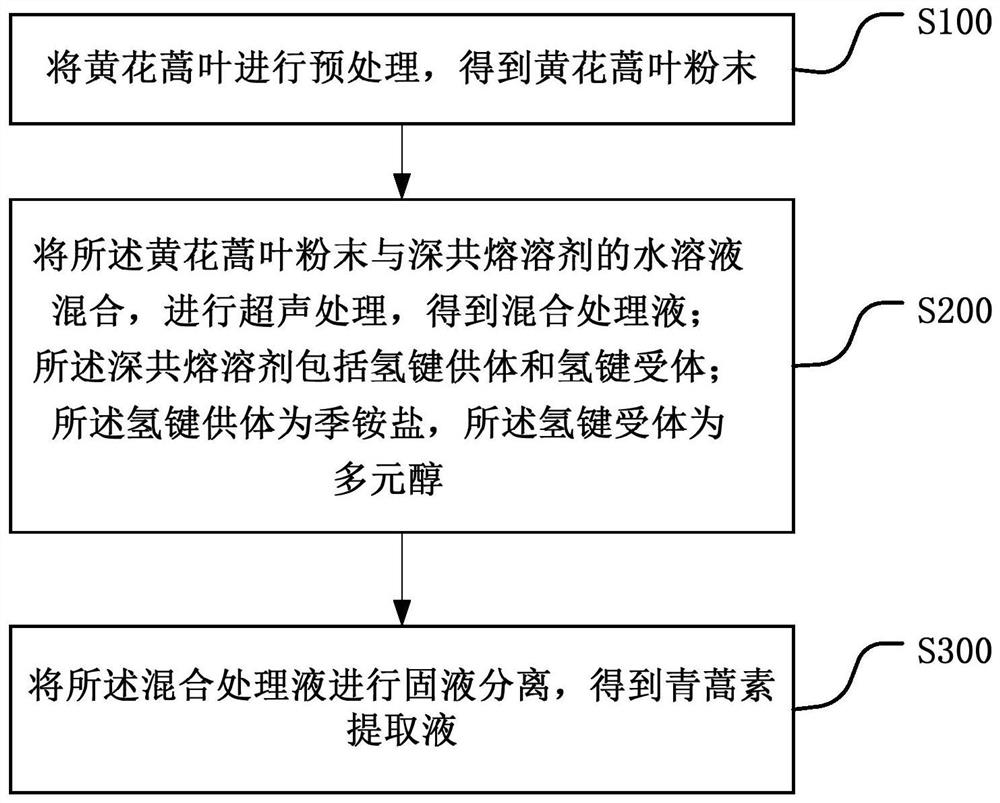
Method for ultrasonically extracting artemisinin by using deep eutectic solvent
A deep eutectic solvent, artemisinin technology, applied in organic chemistry and other directions, can solve the problems of high extraction cost, easy destruction of active ingredients, environmental pollution, etc., and achieve the effects of improving extraction efficiency, protecting the environment, and simplifying the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] (1) Preparation of choline chloride-isosorbide deep eutectic solvent (ChCl-Isos DES)
[0048] Weigh 1.396 g of Choline chloride (abbreviated as ChCl) and 2.923 g of isosorbide (abbreviated as Isos), add them into the sample bottle at a molar ratio of 1:2 and mix well. At 50°C, the stirring speed was 500rpm magnetic stirring for 10min; then the ultrasonic power was 1000W, and the ultrasonic temperature was 50°C for ultrasonication for 5min; so circulated until a transparent and clear choline chloride-isosorbide deep eutectic solvent ( ChCl-Isos DES).
[0049] (2) Characterization of physicochemical properties of choline chloride-isosorbide deep eutectic solvent (ChCl-Isos DES)
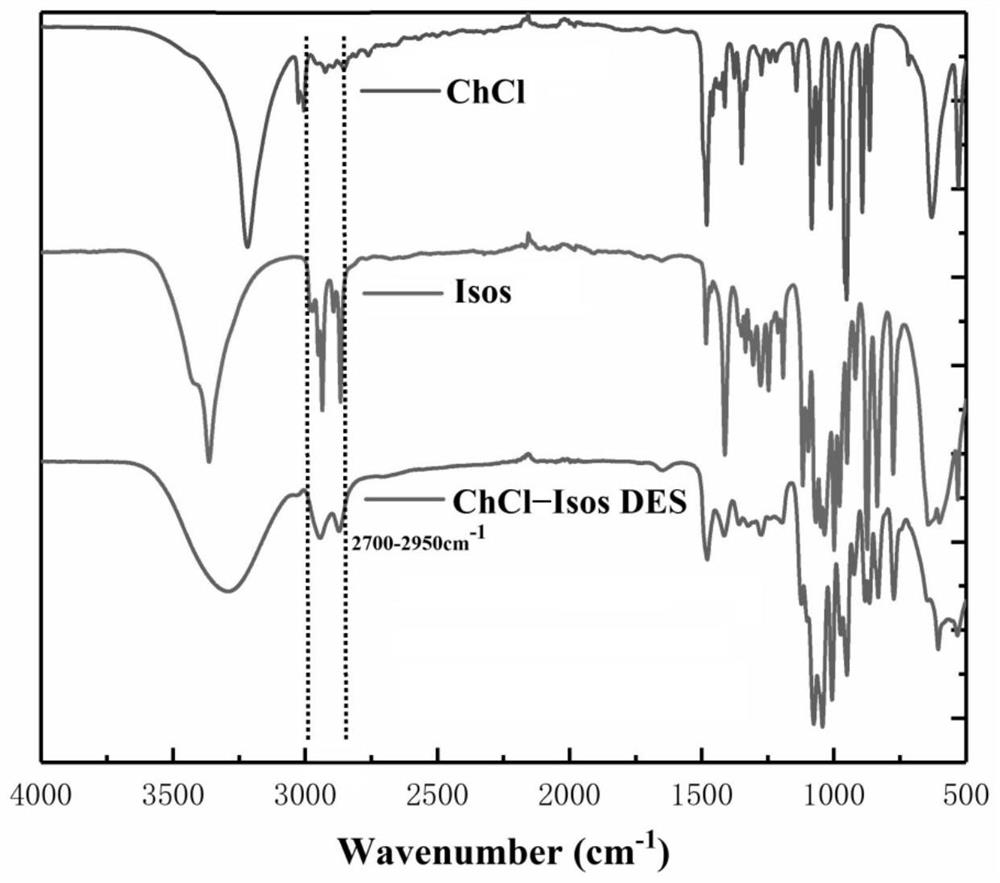
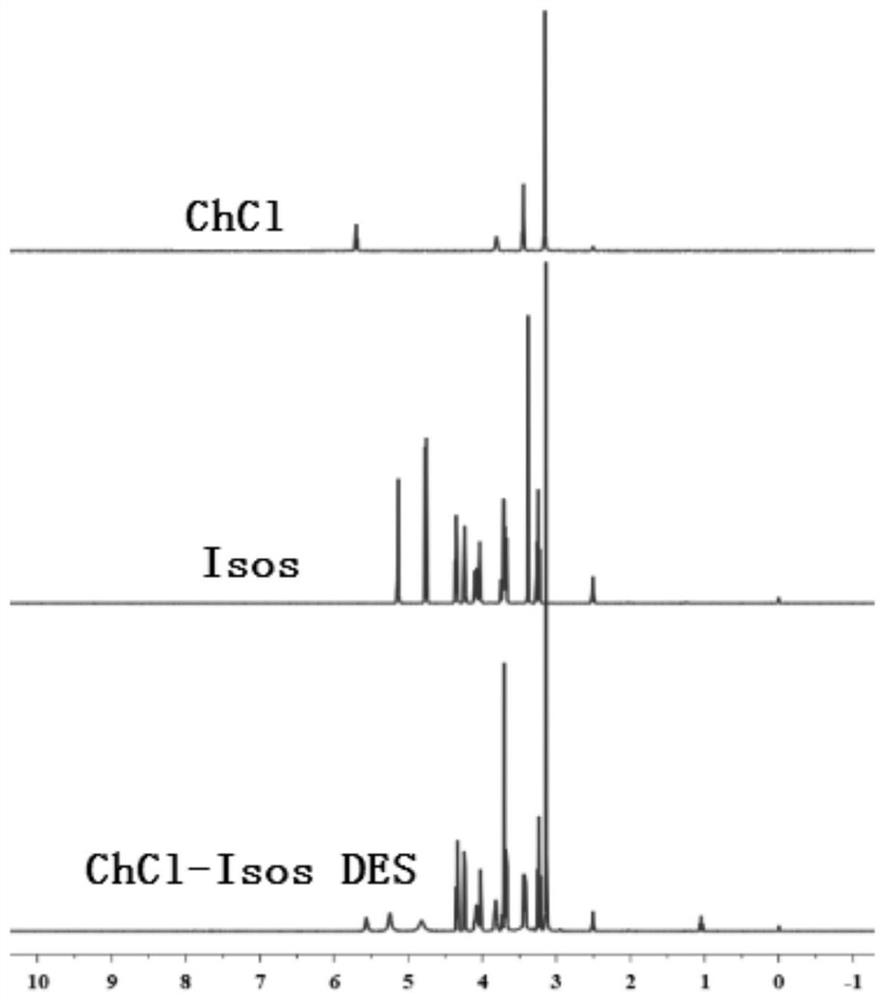
[0050] Measured ChCl, Isos, ChCl-Isos DES Fourier transform infrared spectrum (FTIR) contrast such as figure 2 Shown, known, ChCl-Isos DES chemical bond position and chemical group are all different with ChCl, Isos; Use DMSO as deuterated solvent, record the proton nuclear magnetic resonance s...
Embodiment 2
[0056] Example 2 Ultrasonic extraction of artemisinin using ChCl-Isos DES (molar ratio is 1:2)
[0057] (1) Take the oil of Artemisia annua, remove the branches, take the leaves and place them in a blast drying oven at 60°C to air-dry until there is no water residue in the leaves of Artemisia annua, crush and grind them with a mortar, and pass through a 120-mesh sieve to obtain 120-mesh yellow flowers Artemisia leaf powder for later use.
[0058] (2) Mix 0.1g of Artemisia annua leaf powder with 2mL of ChCl-Isos DES (1:2) aqueous solution (the ratio of ChCl-Isos DES (1:2) to water is 0.6g:1mL), and the input power of ultrasound is 1000W, ultrasonic frequency 40kHz, ultrasonic at 45°C for 30min to obtain a mixed treatment solution.
[0059] (3) The mixed treatment solution was centrifuged at 1300 rpm for 6 minutes to obtain an artemisinin extract.
[0060] (4) Take 1 mL of artemisinin extract, add 0.2 wt% NaOH solution, react in 50°C water bath for 30 min, cool to room tempera...
Embodiment 3
[0061] Example 3 Test the ChCl-Isos DES formed by different molar ratios of ChCl and Isos on the extraction effect of artemisinin
[0062] (1) Prepare two ChCl-Isos DES with different molar ratios of ChCl and Isos (1:4, 1:6)
[0063] The preparation steps are the same as the step (1) of Example 1, except that ChCl and Isos are mixed in a molar ratio of 1:4 and 1:6 respectively, and the obtained deep eutectic solvent is respectively denoted as ChCl-Isos DES (1 :4), ChCl-Isos DES (1:6), the deep eutectic solvent that embodiment 1 obtains is denoted as ChCl-Isos DES (1:2).
[0064] (2) The ChCl-Isos DES prepared by different molar ratios of ChCl and Isos is tested for the extraction performance of artemisinin
[0065] (2.1) The extraction steps of artemisinin were the same as in Example 2, replacing ChCl-Isos DES (1:2) with ChCl-Isos DES (1:4) and ChCl-Isos DES (1:6) respectively.
[0066] (2.2) The determination steps of the extraction efficiency of artemisinin are the same as...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Freezing point | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com