Preparation method of bis(2,4,6-trimethylbenzoyl)phenyl phosphine oxide
A technology of trimethylbenzoyl and phenyl phosphine oxide, which is applied in the field of preparation of bisphenyl phosphine oxide, can solve problems such as unfavorable safety, inability to recover, increase by-products, etc., and achieve better inhibition effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
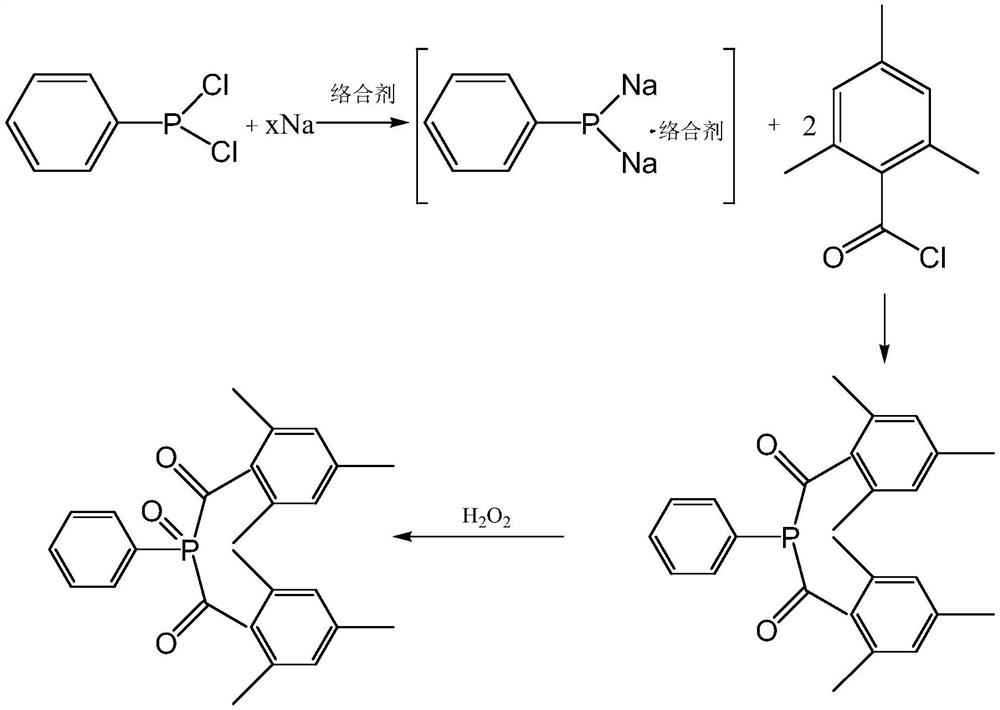
[0097] This embodiment provides a preparation method of bis(2,4,6-trimethylbenzoyl)phenylphosphine oxide, comprising:
[0098] (1) In a 500mL four-necked bottle, add 11g of metallic sodium and 150g of toluene, heat up to 100°C under nitrogen protection, and stir for 3 hours to beat the metallic sodium into sodium sand to obtain a sodium sand suspension;
[0099] (2) Keep the reflux state, add 21g of phenylphosphine dichloride dropwise to the sodium sand suspension in step (1), add it in about 2h, keep it warm and reflux for 4h, until the reaction solution becomes bright yellow, then in the reflux state Add 4.2g (0.5eq) tetrahydrofuran, drop it for about 0.5h, keep it warm and reflux for 2h, lower the temperature to 70°C under the protection of nitrogen, add 43g of m-trimethylbenzoyl chloride dropwise, control the reaction temperature at 75°C, and finish adding in about 2h, 75 ℃ heat preservation reaction for 8h;
[0100] (3) After the reaction in step (2) ends, add 120g of wa...
Embodiment 2
[0104] This embodiment provides a preparation method of bis(2,4,6-trimethylbenzoyl)phenylphosphine oxide, comprising:
[0105] (1) In a 500mL four-necked bottle, add 11g of sodium metal and 150g of toluene, add 4.2g (0.5eq) of tetrahydrofuran, heat up to 100°C under nitrogen protection, and stir for 3 hours to beat the sodium metal into sodium sand to obtain a sodium sand suspension ;
[0106] (2) Keep the reflux state, add dropwise 21g phenylphosphine dichloride in the sodium sand suspension in step (1), add about 2h, insulate and reflux for 4h, until the reaction solution becomes bright yellow, nitrogen protection lowers the temperature to At 70°C, add 43g of mesitylene benzoyl chloride dropwise, control the reaction temperature at 75°C, finish adding in about 2 hours, and keep the reaction at 75°C for 8 hours;
[0107] (3) After the reaction in step (2) ends, add 120g of water dropwise to the reaction solution, stir at room temperature for 0.5h, let stand to separate liqui...
Embodiment 3
[0112] This embodiment provides a preparation method of bis(2,4,6-trimethylbenzoyl)phenylphosphine oxide, comprising:
[0113] (1) In a 500mL four-necked bottle, add 20g of metallic sodium and 150g of toluene, heat up to 100°C under nitrogen protection, and stir for 2 hours to beat the sodium into sodium sand to obtain a sodium sand suspension;
[0114] (2) Keep the reflux state, add 21g of phenylphosphine dichloride dropwise to the sodium sand suspension, add about 2h, keep warm and reflux for 6h, until the reaction solution turns bright yellow, add 1.7g of tetrahydrofuran (0.2 eq), about 0.5h after dripping, heat preservation and reflux for 4h, under nitrogen protection, lower the temperature to 70°C, add 43g of mesitylene benzoyl chloride dropwise, control the reaction temperature at 70°C, finish adding about 2h, and keep the temperature at 70°C for 10h;
[0115] (3) After the reaction finishes, 120g of water is added dropwise to the reaction solution, stirred at room tempe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com