Organic compound, scintillator material and preparation method and application thereof
A technology of organic compounds and scintillator materials, applied in the direction of organic chemistry, chemical instruments and methods, luminescent materials, etc., can solve the problems of restricting wide application, and achieve the effect of improving utilization rate, improving luminous performance and luminescent stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] An organic compound M001, the structural formula is as follows:
[0091]
[0092] The preparation method of this organic compound M001 comprises the steps:
[0093] (1)
[0094] Weigh 5 g of carbazole, add it to 40 mL of dry tetrahydrofuran, add 20.5 mL of n-hexane solution with a concentration of 1.6 mol / L n-butyllithium dropwise in an ice bath, and react at room temperature for 2 hours to obtain a mixed solution; Add 10 mL of a tetrahydrofuran solution of trichlorotriazine with a concentration of 0.44 mol / L dropwise into the solution, and react at 35° C. for 12 hours. After the reaction, filter with suction to obtain a solid crude product, wash the solid crude product with ice acetone, and obtain White solid CzDClT.
[0095] (2)
[0096] Slowly add 15 mL of a tetrahydrofuran solution of o-iodophenol with a concentration of 0.67 mol / L to 4.66 mL of an aqueous sodium hydroxide solution with a concentration of 3 mol / L, stir and react at room temperature for 1 h...
Embodiment 2
[0102] A kind of organic compound M002, structural formula is as follows:
[0103]
[0104] The difference between the preparation method of the organic compound M002 and Example 1 is that the o-iodophenol in step (2) is replaced with an equimolar amount of m-iodophenol, and the amounts of other components and experimental conditions are the same as in Example 1.
[0105] The organic compound M002 is characterized as follows:
[0106] 1 H NMR (400MHz, CDCl 3 ): δ8.72 (dd, 4H), 8.09-8.00 (m, 5H), 7.58 (td, 1H), 7.47–7.35 (m, 9H), 7.22 (td, 1H).
[0107] 13 C NMR (101MHz, CDCl 3 ): δ171.35, 165.63, 153.10, 140.07, 138.86, 130.14, 128.03, 127.08, 126.76, 123.74, 119.74, 118.02, 91.51.
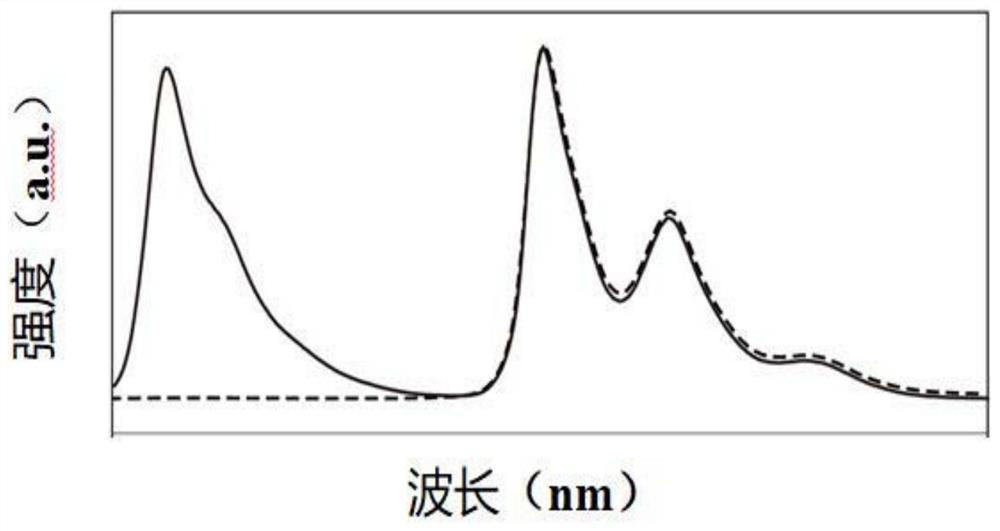
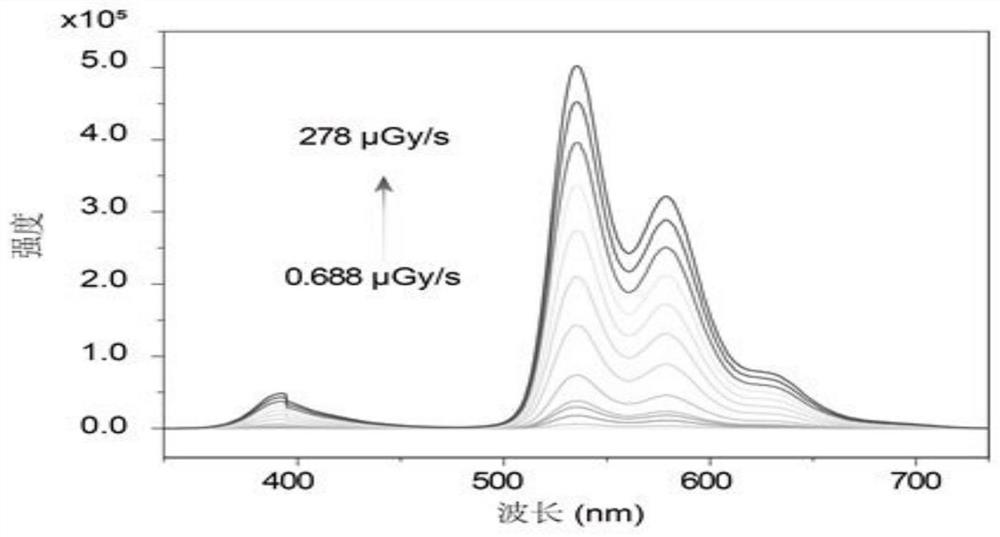
[0108] The organic compound obtained in this embodiment is detected by the same detection method as in Example 1, and the obtained organic compound M002 has an ultraviolet excitation emission spectrum, an X-ray excitation emission spectrum, and an emission spectrum under different doses of...
Embodiment 3
[0110] A kind of organic compound M003, structural formula is as follows:
[0111]
[0112] The difference between the preparation method of the organic compound M003 and Example 1 is that the o-iodophenol in step (2) is replaced by an equimolar amount of p-iodophenol, and the amounts of other components and experimental conditions are the same as in Example 1.
[0113] The organic compound M003 is characterized as follows:
[0114] 1 H NMR (400MHz, CDCl 3 ):δ8.70(dd,J=6.4,2.9Hz,4H),8.06–7.99(m,4H),7.93–7.88(m,2H),7.44–7.37(m,8H),7.20–7.14(m ,2H).
[0115] 13 C NMR (101MHz, CDCl 3 ): δ171.89, 165.54, 152.52, 139.20, 138.79, 127.13, 126.76, 124.87, 123.82, 119.78, 117.98, 90.36.
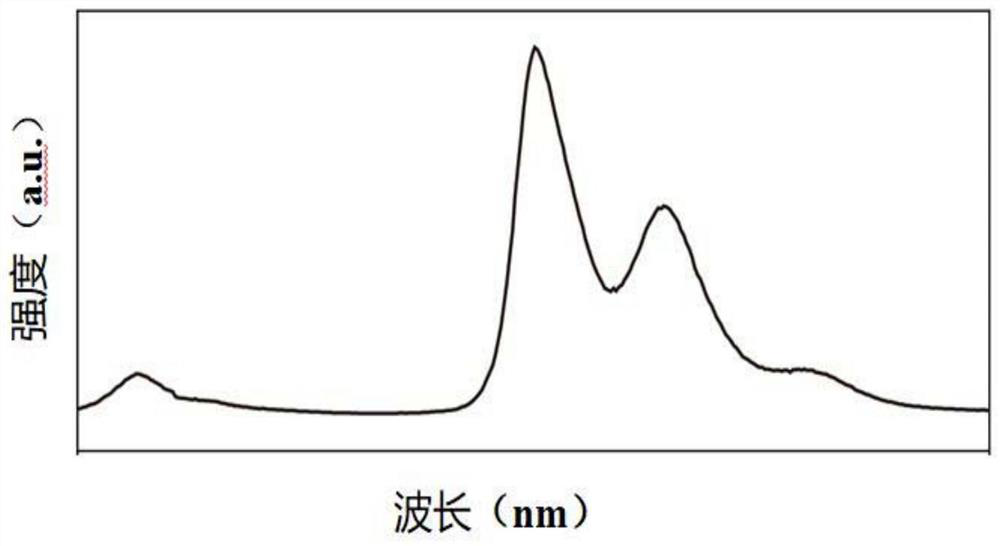
[0116] The organic compound M003 obtained in this example was detected by the detection method described in Example 1, and the ultraviolet excitation emission spectrum, X-ray excitation emission spectrum, and emission spectrum under different doses of X-ray excitation of the organic compound ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com