A method for the electrocatalytic synthesis of aromatic nitriles using aromatic methyl compounds as raw materials
A compound, aromatic nitrile technology, applied in the field of electrocatalytic synthesis of aromatic nitrile, to achieve the effect of preventing substrate polymerization and electrode passivation, simple operation and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: the preparation of p-methoxybenzonitrile (formula (2-1)):
[0040] Add 0.0244g p-methoxytoluene, 0.0048g electrocatalyst 3c, 0.0492g hydroxylamine sulfate, 0.3419g tetrabutylammonium perchlorate, 8mL solvent (CH 3 CN:CH 2 Cl 2 =4:1), 2mL distilled water, 50℃, Pt as anode, Pt as cathode, Ag / AgNO 3 (0.01M AgNO 3 Acetonitrile solution) electrode was used as a reference electrode, and electrolyzed at a constant potential of 1.5V for 12h to obtain the product p-methoxybenzonitrile. The yield of electrolysis products was analyzed by gas chromatography GC, and the analysis method was the area normalization method. The product yield is 93% as shown in Table 1. The reaction solution was extracted with anhydrous ether (5mL×3), dried over anhydrous sodium sulfate, purified by column chromatography (petroleum ether / ethyl acetate=15:1), and then subjected to structural characterization.
[0041] The product is characterized by: 1 HNMR (500MHz, CDCl 3 )δ: 3.86(s...
Embodiment 2-5
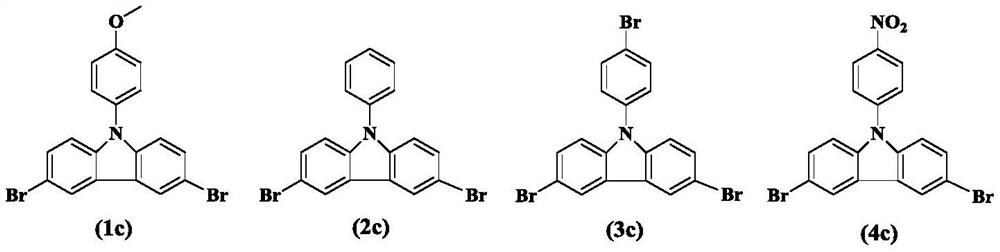
[0042]Embodiment 2-5: The reaction steps are the same as the reaction process of Example 1, the difference is that the electrocatalyst is replaced by equimolar 0.0033g 1c, 0.0040g 2c, 0.0045g 4c and no electrocatalyst is added, and the above constant potential is carried out Electrolysis experiments, the results are listed in Table 1.
[0043] Table 1 Electrolysis of p-methoxytoluene under the action of different electrocatalysts to generate p-methoxybenzonitrile
[0044]
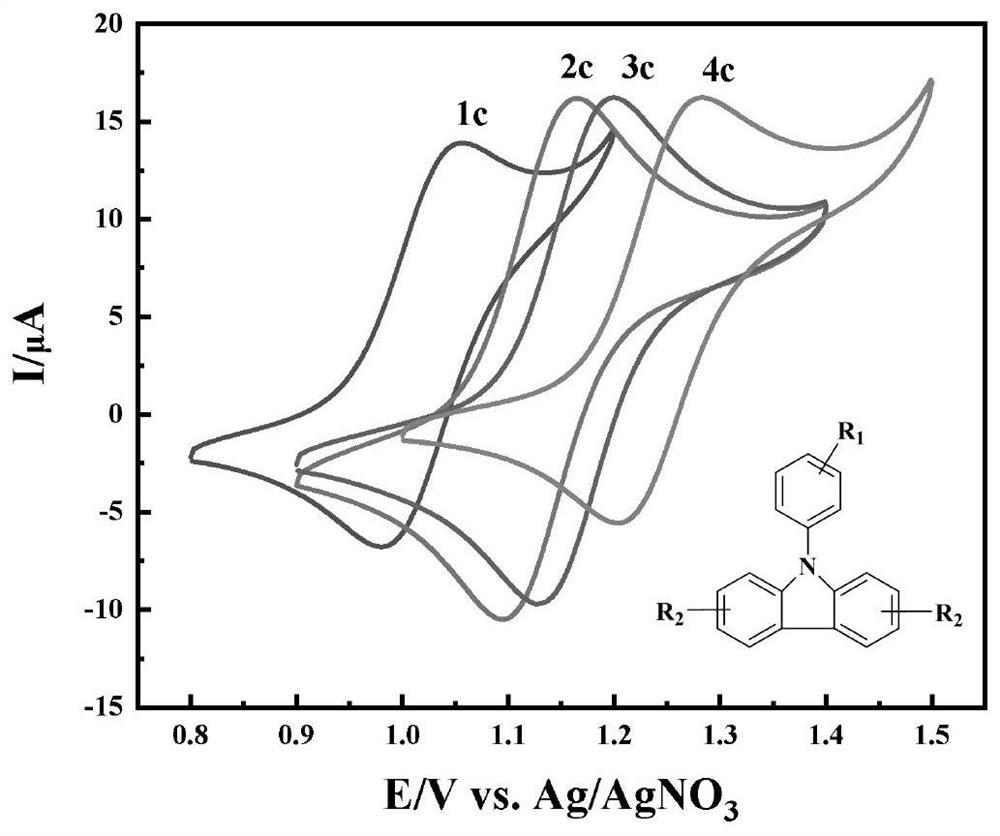
[0045] It can be seen from Table 1 that the yield of aromatic nitriles is 75-93% when electrocatalyst is added, and the yield of aromatic nitriles without electrocatalysis is 50%. It can be seen that electrocatalysts 1c-4c can be used as high-efficiency electrocatalysts for aromatic nitriles. The field of electrocatalytic synthesis of aromatic nitriles from methyl compounds. Due to the low oxidation potential of electrocatalysts 1c and 2c, the catalytic effect in this system is lower than that of 3c, wh...
Embodiment 6
[0046] Embodiment 6: the preparation of p-methoxybenzonitrile (formula (2-1)):
[0047] The difference between the reaction steps and Example 1 is that the electrolyte is 0.1064g lithium perchlorate, and the yield of p-methoxybenzonitrile finally obtained is 68%.
[0048] From the above reaction results, the supporting electrolyte is preferably tetrabutylammonium perchlorate (Example 1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com