Targeted protein degradation c-Met degradation agent as well as preparation method and application thereof
A targeting and solvate technology, applied in the field of medicine, can solve the problems of c-Met small molecule inhibitor acquired drug resistance, etc., achieve significant proliferation inhibitory activity, improve cell selectivity, and good cell selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
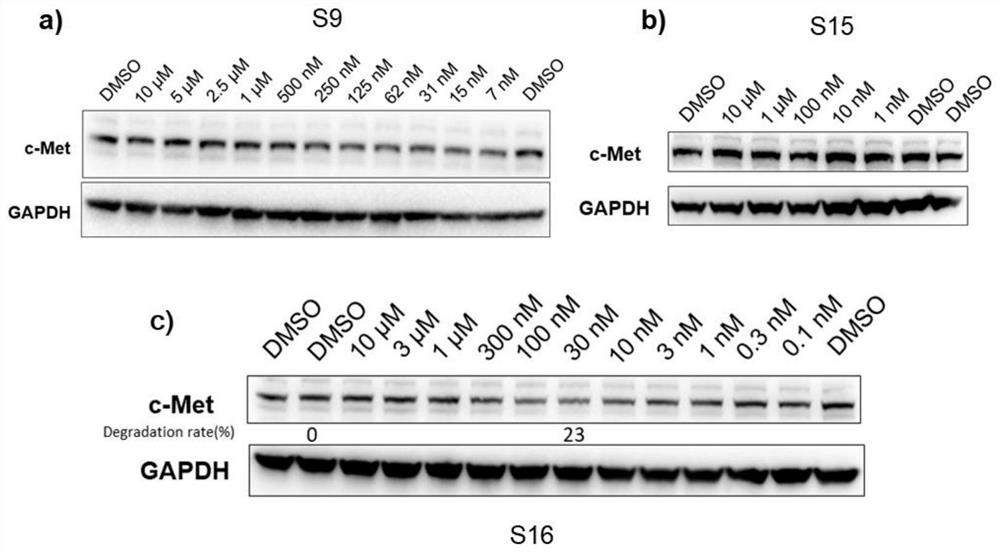
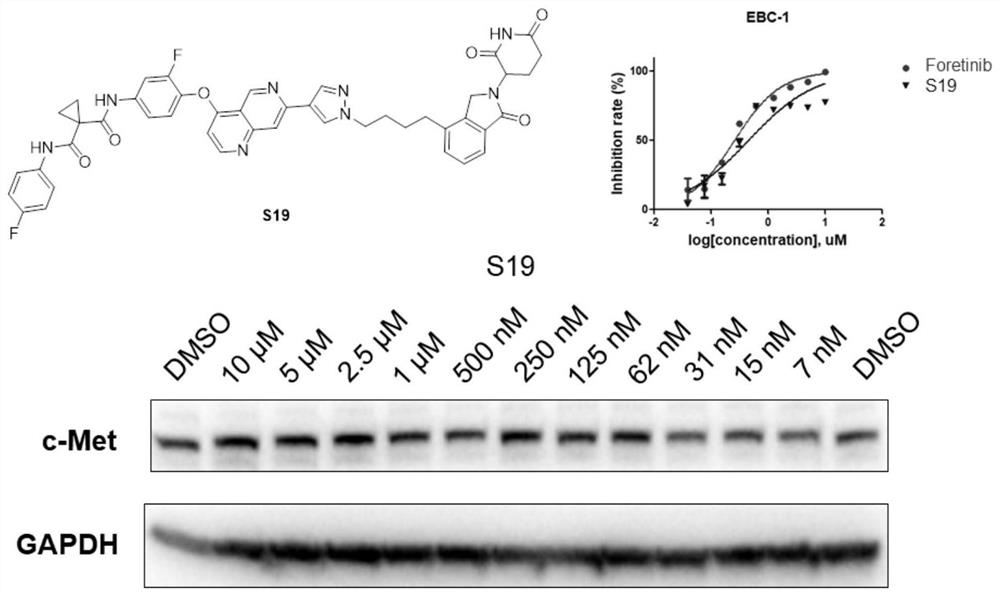
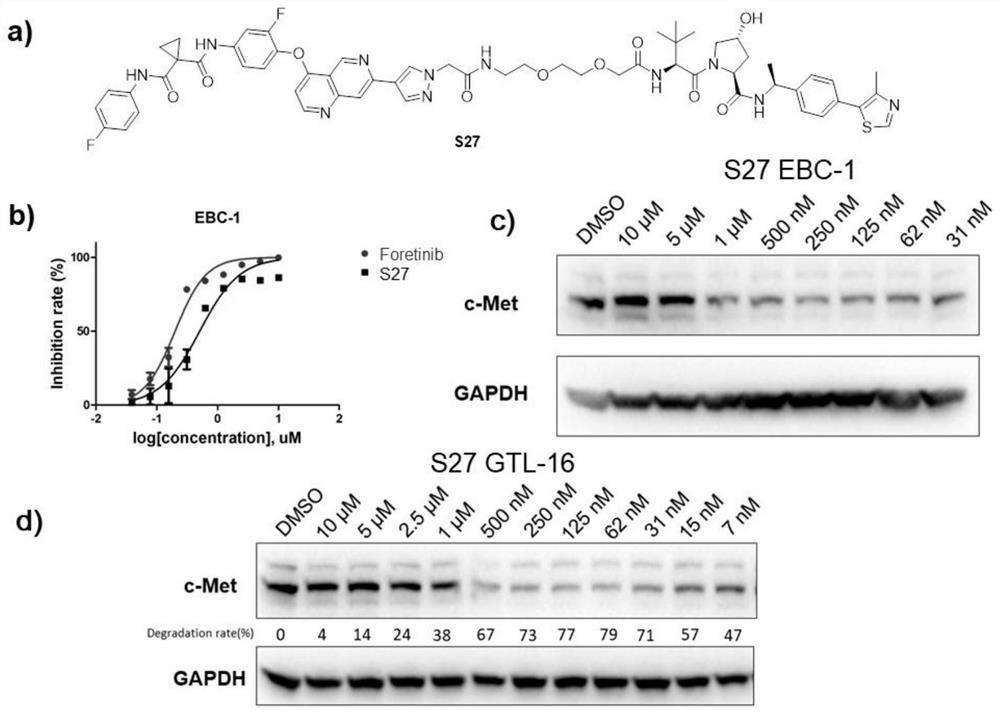
Image
Examples
Embodiment 1
[0044]Example 1: Preparation of 2-(4-(4-(2-fluoro-4-(1-(((4-fluorophenyl)carbamoyl)cyclopropane-1-carboxamido)phenoxy)- 1,6-naphthyridin-7-yl)-1H-pyrazol-1-yl)acetic acid
[0045]
[0046] In the above reaction scheme, the reagents and conditions used are as follows: a) 2-(7-azabenzotriazole)-N,N,N',N'-tetramethyluronium hexafluorophosphate, N, N-diisopropylethylamine, N,N-dimethylformamide; b) hydrogen, 10% wet palladium carbon, ethanol; c) cesium carbonate, N,N-dimethylformamide, 110 ° C; d ) Tetrakis(triphenylphosphine) palladium, cesium carbonate, 1,4-dioxane:water (5:1), 120°C; e) tert-butyl bromoacetate, potassium carbonate, N,N-dimethyl Formamide; f) trifluoroacetic acid, dichloromethane. The reaction steps are as follows:
[0047] Step 1: N-(4-(Benzyloxy)-3-fluorophenyl)-N-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide
[0048]
[0049] Dissolve 1-((4-fluorophenyl)carbamoyl)cyclopropane-1-carboxylic acid (2.9g, 13mmol), 4-(benzyloxy)-3-fluoroaniline (2.82g, 1...
Embodiment 2
[0065] Example 2: Preparation of 2-((2-(2,6-dioxaperidin-3-yl)-1,3-dioxoisoindoline-4-yl)oxy)acetic acid
[0066]
[0067] Reagents and conditions: a) pyridine, 3-amino-2,6-piperidinedione hydrochloride, 110°C; b) tert-butyl bromoacetate, potassium carbonate, N,N-dimethylformamide; c) Trifluoroacetic acid, dichloromethane.
[0068] Step 1: 2-(2,6-dioxopiperidin-3-yl)-4-hydroxyisoindoline-1,3-dione
[0069]
[0070] 4-Hydroxyisobenzofuran-1,3-dione (952mg, 5.84mmol) was dissolved in 10mL of pyridine, and 3-amino-2,6-piperidinedione hydrochloride (960mg, 5.84mmol) was added to react The solution was heated to 110°C and stirred overnight. After the reaction was completed, it was cooled to room temperature, pyridine was distilled off under reduced pressure, and the residue was purified by column chromatography (dichloromethane:methanol=100:1 to 100:3) to obtain a white solid (1.5 g, yield 93%). 1 H NMR (400MHz, DMSO-d 6 )δ11.20(s, 1H), 11.11(s, 1H), 7.66(t, J=7.8Hz, 1H), 7...
Embodiment 3
[0078]
[0079] Reagents and conditions: a) 2-(7-azabenzotriazole)-N,N,N',N'-tetramethyluronium hexafluorophosphate, N,N-diisopropylethylamine, N,N-Dimethylformamide; b) trifluoroacetic acid, dichloromethane; c) 2-(7-azabenzotriazole)-N,N,N',N'-tetramethylurea Hexafluorophosphate, N,N-Diisopropylethylamine, N,N-Dimethylformamide.
[0080] Step 1: tert-butyl(2-(2-((2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindolyl-4-yl)oxy) Acetylamino) ethyl) carbamate
[0081]
[0082] 2-((2-(2,6-dioxapiperidin-3-yl)-1,3-dioxoisoindolin-4-yl)oxy)acetic acid (1eq), (2-amino Ethyl) tert-butyl carbamate (1.2eq) was dissolved in 2mL N,N-dimethylformamide, and N,N-diisopropylethylamine (3eq) and 2-(7-aza Benzotriazole)-N,N,N',N'-tetramethyluronium hexafluorophosphate (1.1eq), continue to stir for 1 hour, after the reaction is completed, add 50mL ethyl acetate to dilute, ethyl acetate The phase was washed by adding saturated aqueous sodium bicarbonate solution (50mL*3), and washed by satura...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com