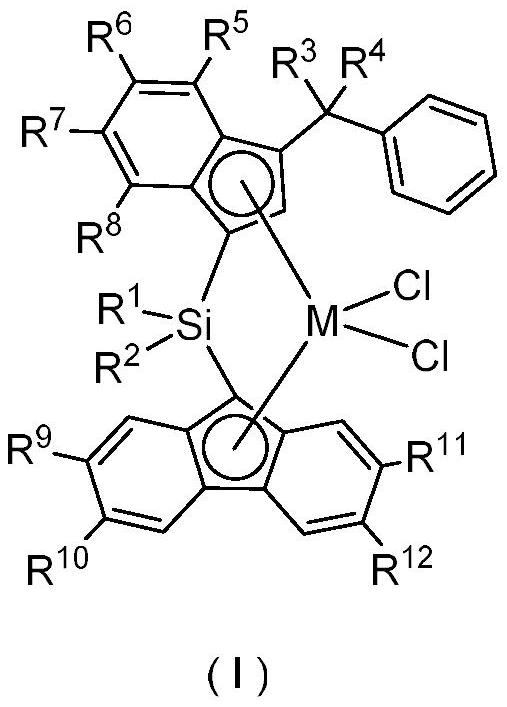
Silicon-based bridged polysubstituted indene-fluorene zirconium and hafnium complex and application thereof in olefin high-temperature polymerization
A technology for olefin polymerization and complexes, which is applied in the field of silicon-based bridged multi-substituted indene-fluorene zirconium and hafnium complexes and its high-temperature polymerization of olefins, which can solve the problems of decomposition and deactivation of catalyst active species, increased reactivity, loss of activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] The second aspect of the present invention provides the preparation method of the complex provided by the first aspect of the present invention, comprising:
[0052] 1) reacting the compound of formula II with an alkyl alkali metal compound to provide a dialkali metal salt of the compound of formula II;
[0053] 2) reacting a dialkali metal salt of a compound of formula II with a zirconium salt and / or a hafnium salt to provide a compound of formula I;
[0054]
[0055] In the step 1), those skilled in the art can select a suitable alkyl alkali metal compound for the above reaction, for example, the alkyl alkali metal compound can be methyllithium, n-butyllithium, tert-butyllithium, etc. one or a combination of more. The amount of the alkyl alkali metal compound is generally equivalent or excessive relative to the compound of formula II, for example, the molar ratio of the compound of formula II to the alkyl alkali metal compound can be 1:1~5, 1:1.8~2.2 , 1:1~1.5, 1...
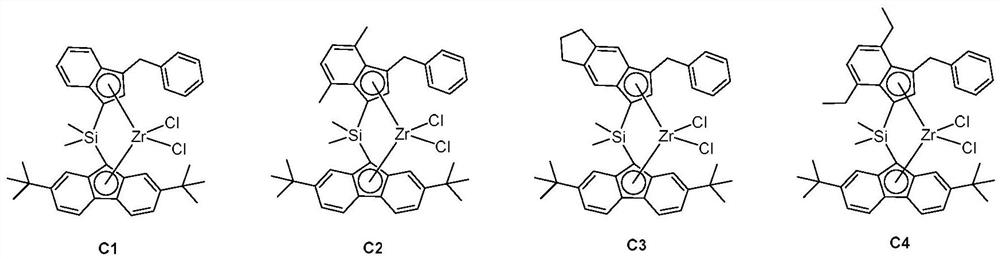
Embodiment 1
[0078] Synthesis of zirconium complex C1
[0079] (1) Synthesis of Ligand Compound L1
[0080]
[0081] Under argon protection, weigh 5.43g (19.5mmol) of 2,7-di-tert-butylfluorene into a Schlenk bottle, add 30mL of ether and 10mL of tetrahydrofuran, and slowly add 9.8mL of n-butyllithium dropwise under ice-salt bath cooling ( 2.0M, 19.6mmol), after the dropping, naturally return to room temperature and stir for 24 hours to obtain the lithium salt of 2,7-di-tert-butylfluorene.
[0082] Under argon protection, weigh 4.03g (19.5mmol) of 3-benzylindene / 1-benzylindene into a Schlenk bottle, add 30mL of ether and 10mL of tetrahydrofuran, and slowly add n-butyllithium 9.8 mL (2.0M, 19.6mmol), after dropping, naturally return to room temperature and stir for 24 hours; through a constant pressure dropping funnel, slowly add 5.03g (39.0mmol) of dimethyldichlorosilane dropwise in an ice-water bath, and drop Afterwards, it was naturally raised to room temperature and continued to sti...
Embodiment 2
[0088] Synthesis of Hafnium Complex C25
[0089] Weigh 1.26g (2.3mmol) of ligand compound L1 in a Schlenk bottle under the protection of argon, add 40mL of ether to dissolve, slowly add 2.3mL of n-butyllithium (2.0M, 4.6mmol) dropwise at -78°C, drop After completion, naturally return to room temperature and stir for 24 hours; at -78°C, add 0.737g (2.3mmol) HfCl 4 ; Naturally rose to room temperature, the reaction system turned into a yellow turbid liquid, stirred and reacted for 48 hours. Remove the solvent in vacuo, add 50 mL of dry dichloromethane, stir for 2 hours and then centrifuge to obtain a red clear liquid, which is concentrated to saturation and recrystallized by adding n-hexane, and placed in a -20°C refrigerator for crystallization and separation to obtain 0.78 g of a yellow solid powder. rate of 42.9%.
[0090] Anal.Calcd.for C 39 h 44 Cl 2 SiHf: C, 59.28; H, 5.61. Found: C, 58.97; H, 5.72%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com