Synthesis method of DLin-MC3-DMA intermediate
A synthesis method and technology of dlin-mc3-dma, which is applied in the field of compound preparation, can solve the problems of harsh production equipment, poor reproducibility, and many by-products of the European Union, and achieve a high total yield, high reproducibility and easy operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
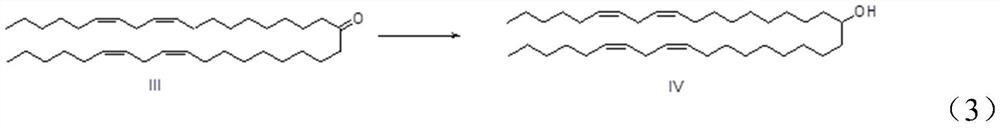
[0028] The synthesis method of (6Z,9Z,28Z,31Z)-heptadecane-6,9,28,31-tetraen-19 alcohol according to the embodiment of the present invention comprises the following steps:
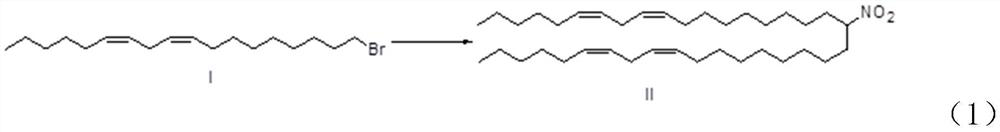
[0029] Step S1, making (6Z,9Z)-18-bromooctadecane-6,9-diene undergo substitution reaction with nitromethane to obtain (6Z,9Z,28Z,31Z)-19-nitro-30 Hepta-6,9,28,31-tetraene.
[0030] Among them, (6Z,9Z)-18-bromooctadecane-6,9-diene, that is, compound I (the compound represented by I in the following reaction formula (1)), undergoes a substitution reaction with nitromethane to form (6Z, 9Z, 28Z, 31Z)-19-nitro-heptadecane-6,9,28,31-tetraene is compound II (the compound shown in II in the following reaction formula (1)), replacing The reaction formula of the reaction is shown in the following formula (1).
[0031]
[0032] Preferably, the substitution reaction occurs in the presence of an organic base comprising LDA, LHMDS, or a mixture thereof. Thus, LDA and LHMDS can well undergo a deprotonation reactio...
Embodiment 1
[0054] (a) Synthesis of compound Ⅱ, namely (6Z,9Z,28Z,31Z)-19-nitro-heptakadecane-6,9,28,31-tetraene
[0055] In a 150mL round bottom flask, 10g of compound I was dissolved in 80mL of tetrahydrofuran, cooled to -5°C, and 0.93g of nitromethane was added. 15.1 mL of 2M LDA was slowly added dropwise; after reacting at -5°C for 3 h, 50 mL of saline solution was added to quench the reaction.
[0056] The organic phase was washed with water, dried and evaporated to dryness; the crude product was purified by column chromatography to obtain 13.1 g of compound II with a yield of 77%.
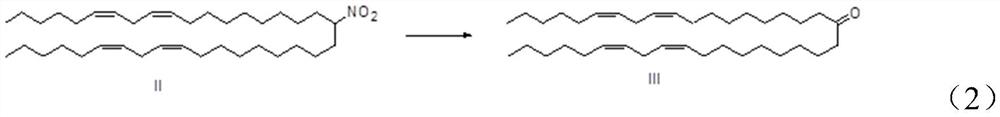
[0057] (b) Synthesis of Compound Ⅲ (6Z, 9Z, 28Z, 31Z)-Heptadecane-6,9,28,31-tetraen-19-one
[0058] In a 150 mL round bottom flask, add 13 g of the above compound II into 40 mL of tetrahydrofuran and 10 mL of water, add 1.86 g of sodium hydroxide, and react at 50° C. for 4 h.
[0059] Tetrahydrofuran was distilled off, and 70 mL of dichloromethane was added for extraction. The organic phase was washed ...
Embodiment 2
[0063] (a) Synthesis of compound Ⅱ, namely (6Z,9Z,28Z,31Z)-19-nitro-heptakadecane-6,9,28,31-tetraene
[0064] In a 150mL round bottom flask, 10g of compound I was dissolved in 80mL of tetrahydrofuran, cooled to -5°C, and 0.93g of nitromethane was added. 15.1 mL of 2M LHMDS was slowly added dropwise; after reacting at -5°C for 6 h, 50 mL of saline solution was added to quench the reaction.
[0065] The organic phase was washed with water, dried and evaporated to dryness; the crude product was purified by column chromatography to obtain 13.1 g of compound II with a yield of 72%.
[0066] (b) Synthesis of Compound Ⅲ (6Z, 9Z, 28Z, 31Z)-Heptadecane-6,9,28,31-tetraen-19-one
[0067] In a 150mL round bottom flask, add 13g of compound II into 40mL of tetrahydrofuran and 10mL of water, add 2.61g of potassium hydroxide, and react at 60°C for 4h.
[0068] Tetrahydrofuran was distilled off, and 70 mL of dichloromethane was added for extraction. The organic phase was washed once more wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com