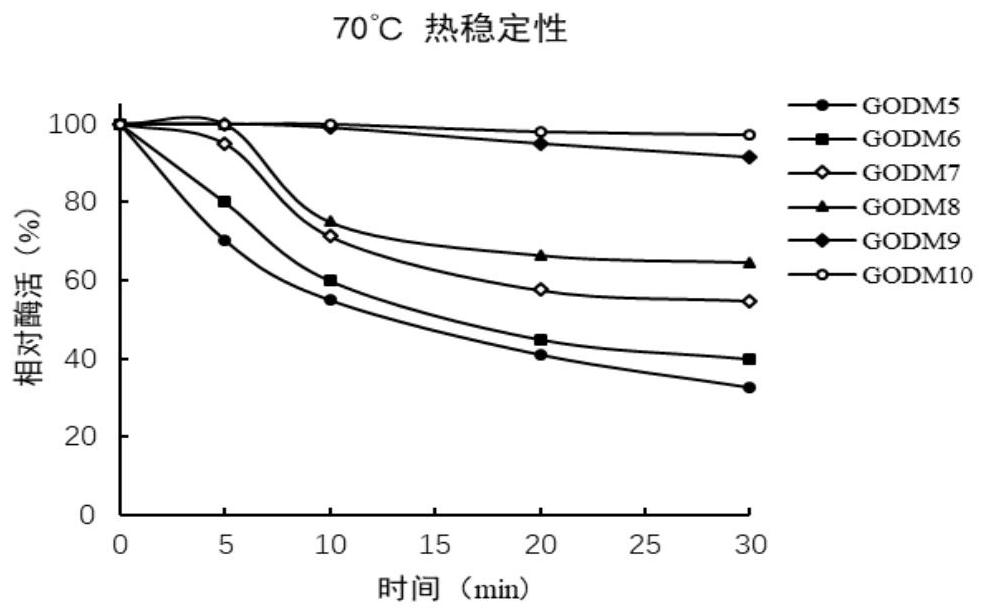
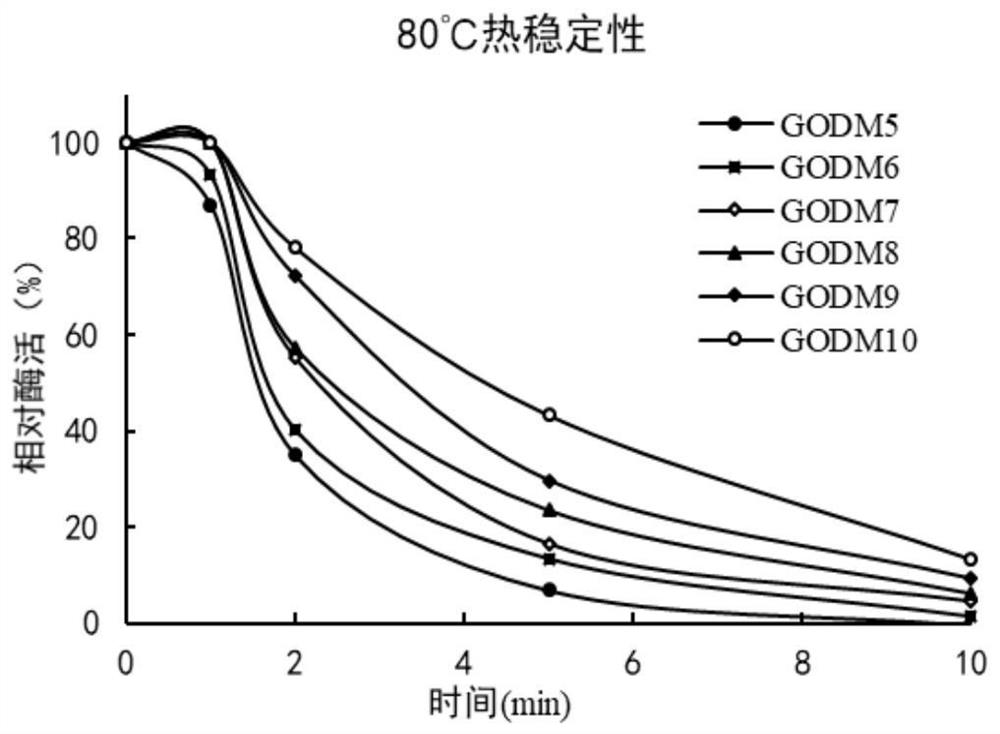
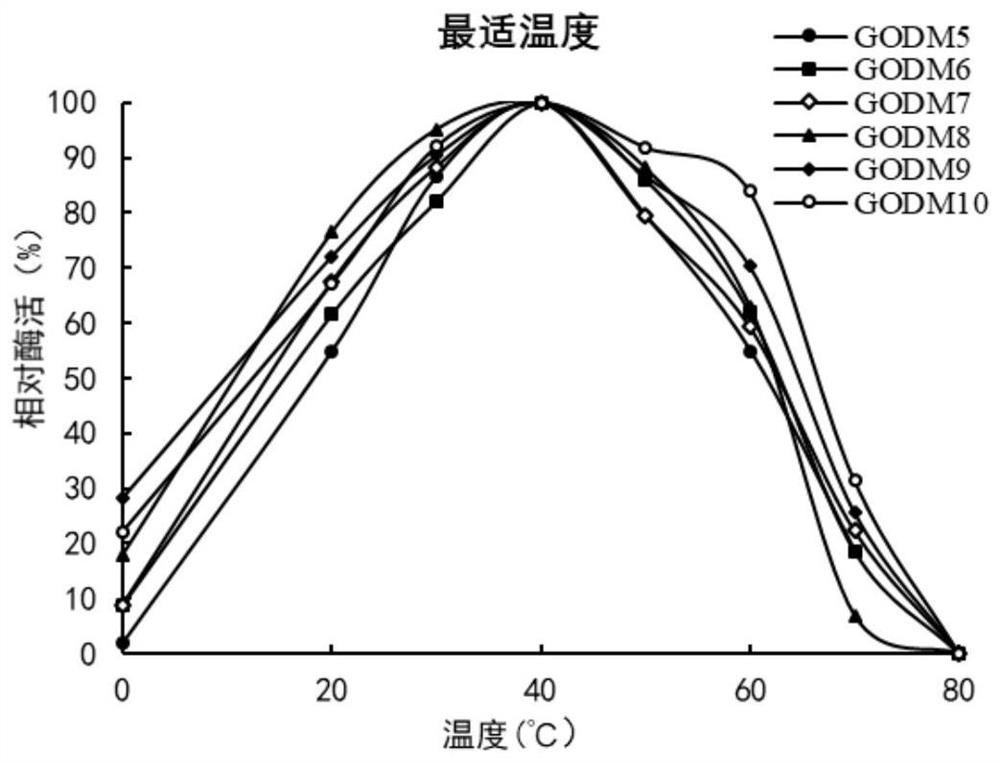
Glucose oxidase mutant GOD with improved thermal stability, and gene and application thereof
A technology of glucose oxidase and heat stability, applied in the field of genetic engineering, can solve the problems of low enzyme activity, low yield, and restriction of industrialization development, and achieve the effects of good heat stability, broad application prospects, and high catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Site-directed mutation of glucose oxidase
[0037] The glucose oxidase gene godm5 is the fifth-generation mutant of the wild type god cloned from Aspergillus niger, and the mutation process is as follows:
[0038]Using the glucose oxidase GOD derived from Aspergillus niger as the parent, the 82nd Glu of the wild-type glucose oxidase GOD was mutated to Cys to obtain the mutant GOD-M1; the 418th Val of the mutant GOD-M1 was mutated into Glu, the mutant GOD-M2 was obtained; the 508th Asn of the mutant GOD-M2 was mutated into His, and the mutant GOD-M3 was obtained; the 32nd Thr of the mutant GOD-M3 was mutated into Val, and the mutant GOD-M3 was obtained Mutant GOD-M4; the 313th Asp of the mutant GOD-M4 was mutated into Lys to obtain the mutant GOD-M5, the amino acid sequence of GOD-M5 is shown in SEQ ID NO.1.
[0039] Using the recombinant plasmid pPIC9-godm5 sequence of GOD-M5 as a template, according to the optimized codon table of Pichia pastoris, the 68th A...
Embodiment 2
[0043] The construction of embodiment 2 glucose oxidase engineering strain
[0044] (1) Construction of expression vector and expression in yeast
[0045] Using the glucose oxidase recombinant plasmid pPIC9-godm5 as a template, the mutants were amplified using site-directed mutagenesis reagents. After verification by nucleic acid gel, add 1 μL of DMT enzyme to the PCR product, mix well and incubate at 37°C for 1 hour. Take 2-5 μL of the PCR product digested with DMT enzyme and transform it into DMT competent cells by heat shock. Positive transformants were picked for DNA sequencing, and the transformants with the correct sequence were used to prepare a large number of recombinant plasmids. Linearize expression plasmid vector DNA with restriction endonuclease Bgl II, transform yeast GS115 competent cells by electroporation, culture at 30°C for 2-3 days, pick transformants grown on MD plates for further expression experiments, specific operations Please refer to the Pichia ex...
Embodiment 3
[0046] The preparation of embodiment 3 recombinant glucose oxidase
[0047] (1) Massive expression of glucose oxidase at shake flask level in Pichia pastoris
[0048] The transformant with good thermal stability and high enzyme activity was screened out, inoculated in a 1L Erlenmeyer flask with 300mL of BMGY liquid medium, cultured on a shaking table at 30°C for 48h at 220rpm; centrifuged at 4500rpm for 5min, discarded the supernatant, and poured Add 200 mL of BMMY liquid medium containing 0.5% methanol, induce culture at 30° C., 220 rpm for 48 h. During the induction culture period, methanol solution was added once every 24 hours to compensate for the loss of methanol, so that the methanol concentration was kept at about 0.5%; centrifuged at 12000×g for 10 minutes, the supernatant fermentation liquid was collected, and the enzyme activity was detected and analyzed by SDS-PAGE protein electrophoresis.
[0049] (2) Purification of recombinant glucose oxidase
[0050] Collect ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com