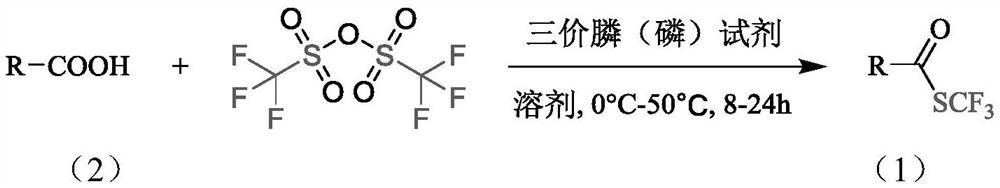
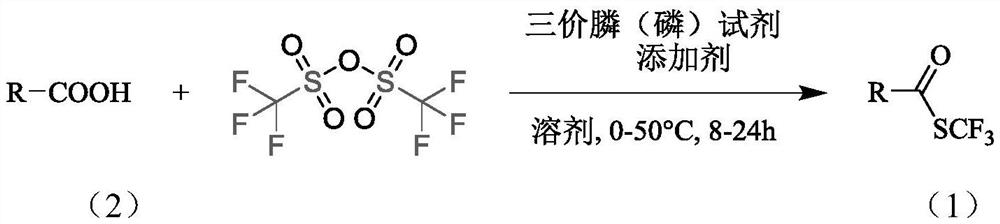
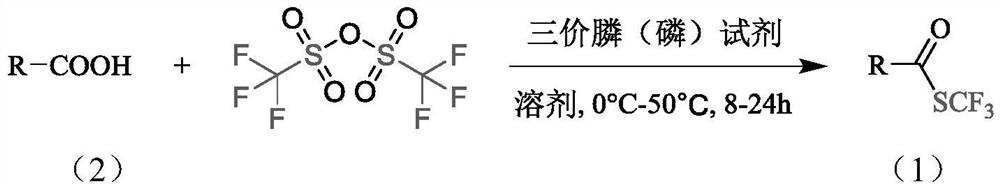
Synthesis method of trifluoromethyl thioester compound
A technology of trifluoromethyl thioester and synthesis method, applied in the direction of organic chemistry, etc., can solve the problems of limited practicability, difficulty in storage, high price, etc., and achieve the effect of reducing synthesis cost, cheap price, and beneficial to industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: In this example, 4-phenylbenzoic acid trifluoromethylthio ester (S-(trifluoromethyl)[1,1'-biphenyl]- 4-carbothioate):
[0031] The reaction equation is:
[0032]
[0033]The synthesis steps and process are: add 4-phenylbenzoic acid (0.4mmol, 79.2mg), triphenylphosphine (2.0mmol, 524mg), tetrabutylammonium bromide ( 1.2mmol, 386mg), then added 4.0mL 1.2-dichloroethane; under inert gas protection, the reaction tube was fixed on a magnetic stirrer, and trifluoromethanesulfonic anhydride (2.0mmol, 564mg) was slowly added under an ice-water bath, After completion, react at 50°C for 12 hours, add appropriate amount of water to the reaction solution, extract with ethyl acetate, dry over anhydrous sodium sulfate, and finally use a rotary evaporator to remove the solvent, and the crude product is subjected to column chromatography (petroleum ether: ethyl acetate Esters=100:1) The target product (1a) was obtained by separation and purification with a yield of 57.7...
Embodiment 2
[0038] Example 2: In this example, 4-bromobenzoic acid trifluoromethylthioester (S-(trifluoromethyl)4-bromobenzothioate) was synthesized by using 4-bromobenzoic acid and trifluoromethanesulfonic anhydride:
[0039] The reaction equation is:
[0040]
[0041] The synthesis steps and process are: add p-bromobenzoic acid (0.4mmol, 80.4mg), triphenylphosphine (2.0mmol, 524mg), tetrabutylammonium iodide (1.2 mmol, 443mg), then add 2.0mL 1.2-dichloroethane; under the protection of inert gas, slowly add trifluoromethanesulfonic anhydride (2.0mmol, 564mg) under the ice-water bath and fix the reaction tube on the magnetic stirrer, room temperature React for 20 hours, add an appropriate amount of water to the reaction solution, extract with ethyl acetate, dry over anhydrous sodium sulfate, and finally use a rotary evaporator to remove the solvent, and the crude product is subjected to column chromatography (petroleum ether:ethyl acetate=500:1 ) separation and purification to obtain ...
Embodiment 3
[0046] Example 3: In this example, the product (S) was synthesized by reacting (S)-(+)-6-methoxy-α-methyl-2-naphthaleneacetic acid (naproxen) with trifluoromethanesulfonic anhydride -(+)-6-Methoxy-α-methyl-2-naphthaleneacetic acid trifluoromethylthio ester (S-(trifluoromethyl)(S)-2-(6-methoxynaphthalen-2-yl)propanethioate):
[0047] The reaction equation is:
[0048]
[0049] The synthesis steps and process are as follows: add (S)-(+)-6-methoxy-α-methyl-2-naphthaleneacetic acid (0.4mmol, 92mg), triphenyl phosphine (2.0mmol, 524mg), tetrabutylammonium iodide (1.2mmol, 442mg), then add 4.0mL 1.2-dichloroethane; slowly add trifluoromethanesulfonic anhydride (1.32 mmol, 372 mg), the reaction tube was fixed on a magnetic stirrer, reacted at room temperature for 48 hours, and was detected by TLC. After the reaction was complete, an appropriate amount of water was added to the reaction solution, extracted with ethyl acetate, dried over anhydrous sodium sulfate, and finally The s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com