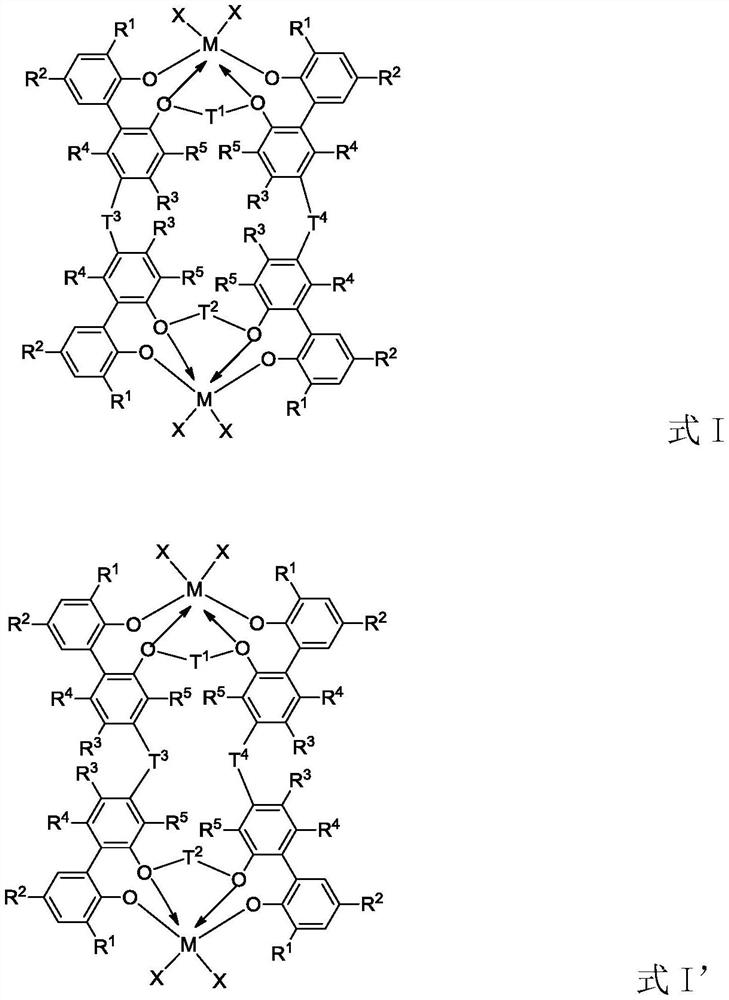
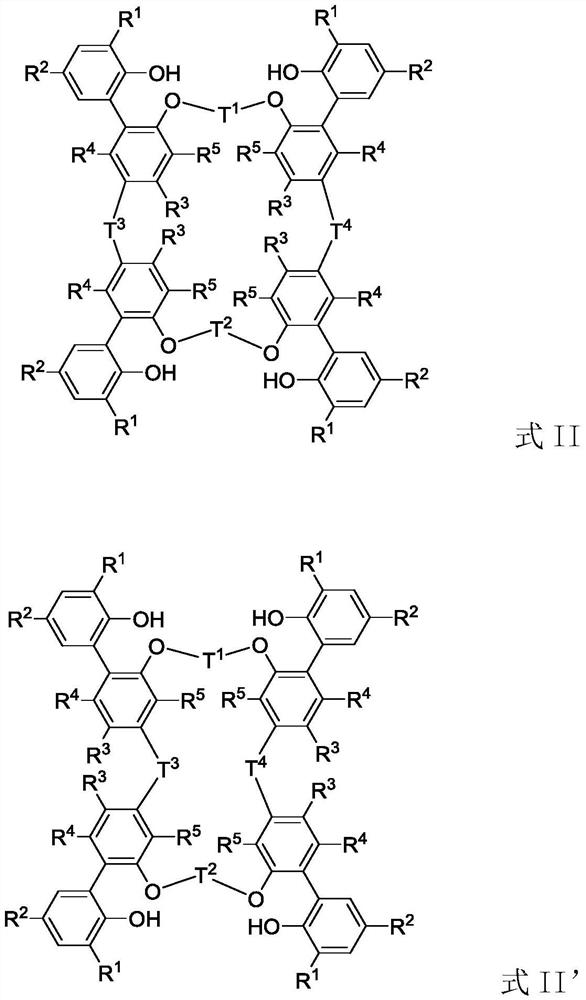
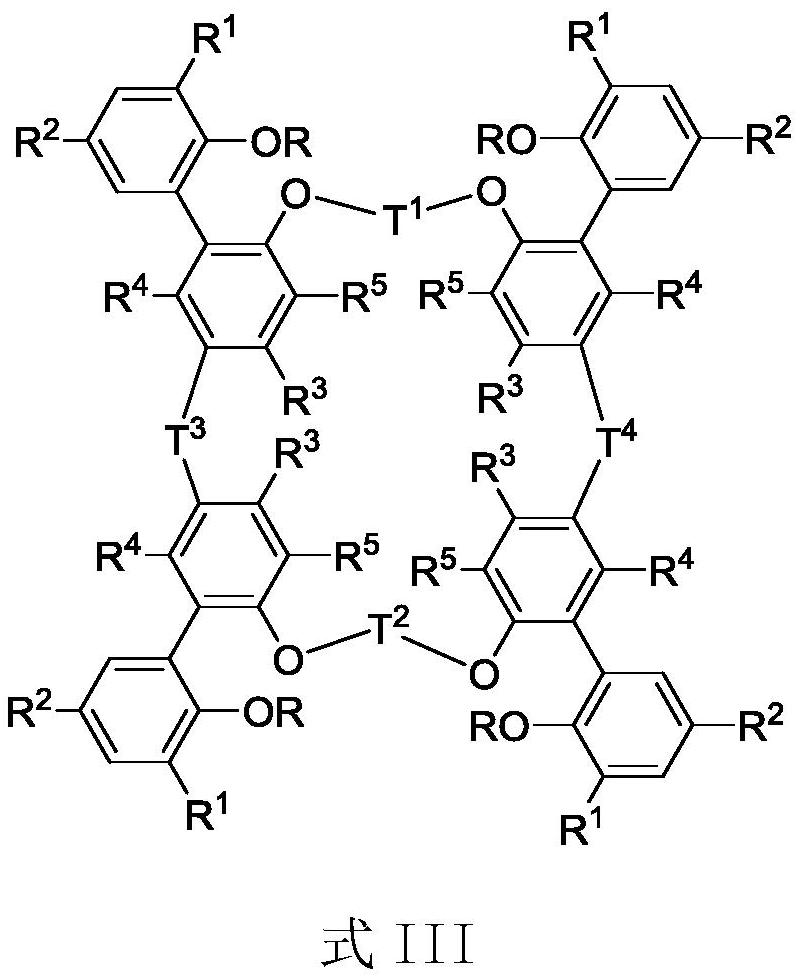
Tetra-aryloxy IVB group binuclear metal complex and preparation method and application of tetra-aryloxy IVB group binuclear metal complex
A group IVB, binuclear metal technology, applied to 4/14 group organic compounds without C-metal bonds, compounds of group 4/14 elements of the periodic table, titanium organic compounds, etc., can solve the problem of low molecular weight of polymerization products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0128] Embodiment 1, the preparation of compound 1-1
[0129] Dissolve 51.02g 4,4'-dichloro-(1,1'-biphenyl)-3,3'-diol (0.2mol) in 500mL acetone, add 40.38g 1,3-dibromopropane (0.2mol , 1.0eq.), and then added 65.16g cesium carbonate (0.2mol, 1.0eq.), heated to 50 ℃ reflux reaction for 12h. The solid was removed by filtration, extracted with ethyl acetate, washed with saturated brine, the organic phases were combined, and dried over anhydrous sodium sulfate. The filtrate was concentrated and purified by silica gel column chromatography (petroleum ether: ethyl acetate = 200:1 (v / v)) to obtain 48.17 g of a colorless solid with a yield of 81.6%.
[0130] The NMR structure of compound 1-1: 1 H NMR (CDCl 3 ,400MHz,TMS):δ7.22-7.19(t,J=8.0Hz,4H),7.13-7.11(d,J=8.0Hz,2H),7.02(s,4H),4.48(t,J=8.0 Hz,8H),2.07(m,4H). 13 C NMR (CDCl 3 ,100MHz,TMS):δ154.05,143.28,128.78,127.78,123.80,114.40,68.43,30.86.
Embodiment 2
[0131] Embodiment 2, the preparation of compound 1-2
[0132] Under a nitrogen atmosphere, 47.22g (0.08mol) of compound 1-1 was dissolved in 300mL of ultra-dry tetrahydrofuran, the temperature of the system was lowered to -78°C, and 200mL of n-butyllithium (0.32mol, 4.0eq., 1.6M) was slowly added dropwise. Hexane solution, react at -78°C for 3h, then slowly add 60.19g of triisopropyl borate (0.32mol, 4.0eq) dropwise, react for another 3h, add 30.0mL of water to quench, concentrate the reaction solution, and extract with ethyl acetate , washed with saturated brine, combined the organic phases, dried over anhydrous sodium sulfate, concentrated the filtrate, added n-hexane to recrystallize and washed, and obtained 37.57 g of white solid with a yield of 74.8%.
[0133] NMR structure of compound 1-2: 1 H NMR (CDCl 3 ,400MHz,TMS):δ8.02(d,J=8.0Hz,4H),7.86(s,4H),7.84(s,4H),7.41(d,J=8.0Hz,4H),6.60(s, 8H), 4.36(t, J=8.0Hz, 8H), 1.93(m, 4H). 13 C NMR (CDCl 3 ,100MHz,TMS):δ159.81,143...
Embodiment 3
[0134] Embodiment 3, the preparation of compound 1-3
[0135] Under a nitrogen atmosphere, 45.82g (0.2mol) of 2-bromo-4-(tert-butyl)phenol was dissolved in 500mL of ultra-dry dichloromethane, then 25.236g of dihydropyran (0.3mol, 1.5eq.) was added and 5.03g of pyridinium p-toluenesulfonate (0.02mol, 0.1eq.) was reacted at 30°C for 6h. Extract with dichloromethane, wash with saturated brine, then combine the organic phases and dry over anhydrous sodium sulfate. The filtrate was concentrated and purified by silica gel column chromatography (petroleum ether: ethyl acetate = 300:1 (v / v)) to obtain 55.50 g of white solid with a yield of 88.6%.
[0136] NMR structures of compounds 1-3: 1 H NMR (CDCl 3 ,400MHz,TMS):δ7.58(d,J=8.0Hz,1H),7.22-7.20(dd,J=8.0Hz,1H),7.04(dt,J=8.0Hz,1H),5.42(m, 1H),3.88–3.84(m,1H),3.55-3.51(m,1H),2.14-2.10(m,1H),2.05-2.03(m,2H),2.02-2.01(m,1H),1.45– 1.40(m,2H),1.22(s,9H). 13 C NMR (CDCl 3 ,100MHz,TMS):δ152.74,144.83,130.36,125.16,114.62,96.23,61.40,34...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com