Direct-compression type organic calcium vitamin D3 chewable tablet and preparation method thereof
A vitamin and organic calcium technology, applied in the direction of organic active ingredients, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of unreasonable daily intake design, affecting the absorption and solubility of other substances Problems such as low absorption rate, to achieve the effect of preventing rickets in young children, high solubility and absorption rate, simple and mature process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
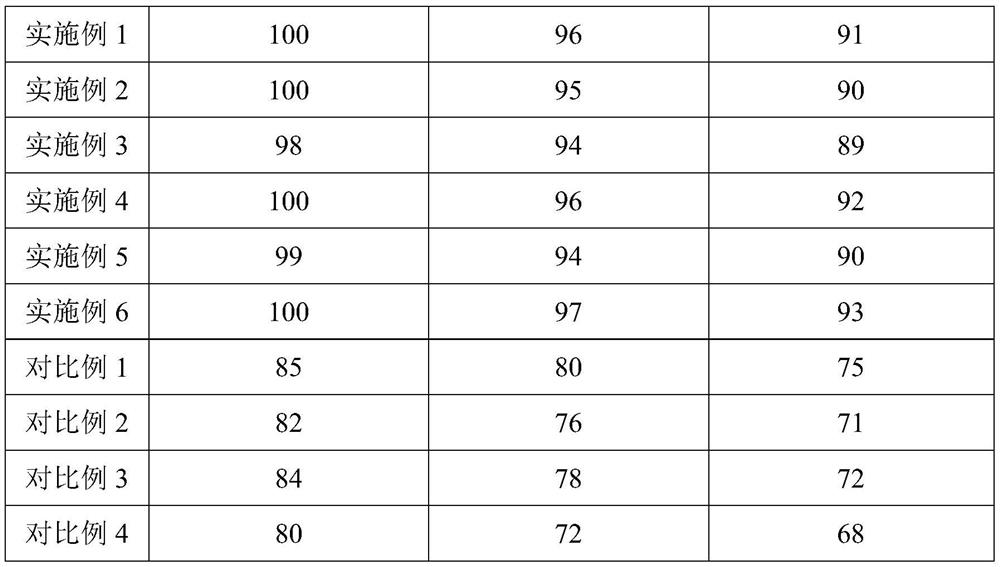
Embodiment 1
[0032] Embodiment 1, a kind of direct compression type organic calcium vitamin D3 chewable tablet
[0033] The direct-compression type organic calcium vitamin D3 chewable tablet is composed of the following ingredients and parts by weight: 50 parts of calcium citrate, 40 parts of L-calcium lactate, 25 parts of microcrystalline cellulose, 1 part of anhydrous glucose, D-mannose 10 parts of sugar alcohol, 1 part of polyethylene glycol 6000, 5 parts of vitamin D3, 0.5 part of polydextrose, 1 part of sucralose, 0.5 part of magnesium stearate.
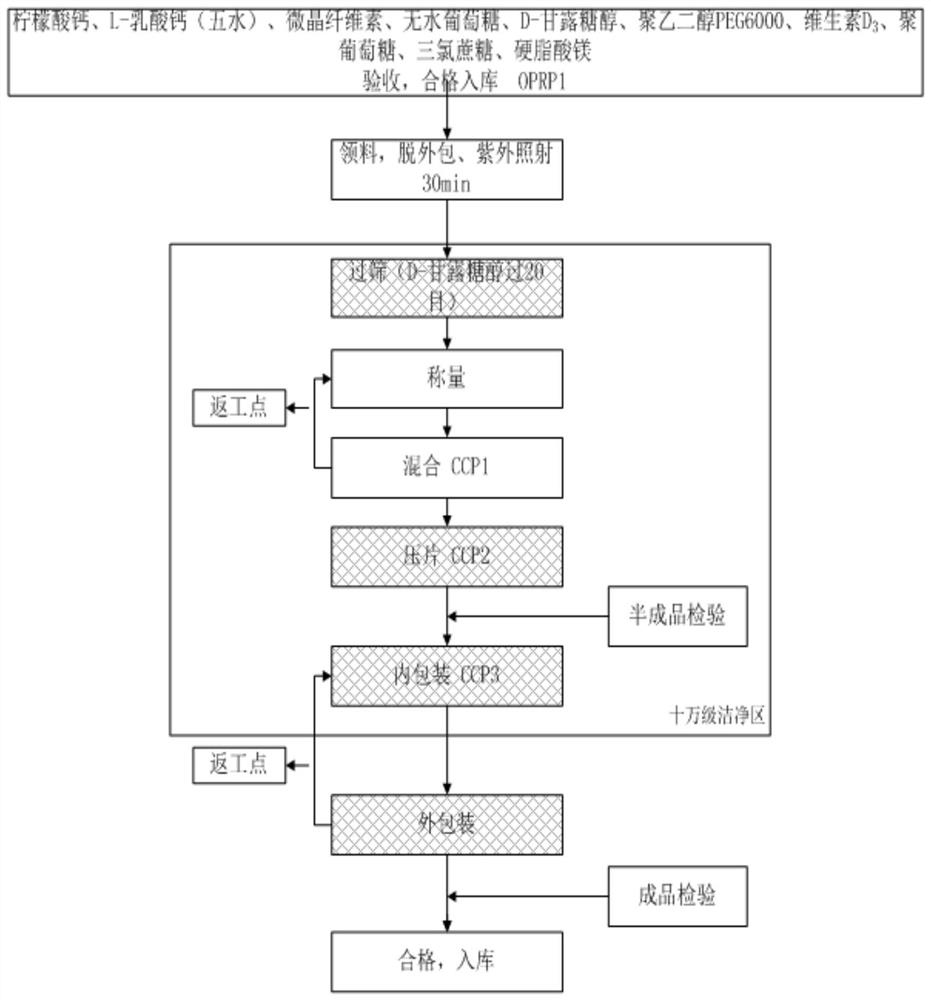
[0034] Such as figure 1 Shown, the preparation method of described direct compression type organic calcium vitamin D3 chewable tablet, comprises the steps:
[0035] S1. Remove calcium citrate, L-calcium lactate, microcrystalline cellulose, anhydrous dextrose, D-mannitol, polyethylene glycol 6000, vitamin D3, polydextrose, sucralose, and magnesium stearate. Packaged and irradiated under UV light for 30 minutes;
[0036] S2, passing D-manni...
Embodiment 2
[0039] Embodiment 2, a kind of direct compression type organic calcium vitamin D3 chewable tablet
[0040] The direct-compression type organic calcium vitamin D3 chewable tablet consists of the following ingredients and parts by weight: 55 parts of calcium citrate, 45 parts of L-calcium lactate, 30 parts of microcrystalline cellulose, 3 parts of anhydrous glucose, D-mannose 15 parts of sugar alcohol, 3 parts of polyethylene glycol 6000, 10 parts of vitamin D3, 0.7 part of polydextrose, 3 parts of sucralose, 0.9 part of magnesium stearate.
[0041] Such as figure 1 Shown, the preparation method of described direct compression type organic calcium vitamin D3 chewable tablet, comprises the steps:
[0042] S1. Remove calcium citrate, L-calcium lactate, microcrystalline cellulose, anhydrous dextrose, D-mannitol, polyethylene glycol 6000, vitamin D3, polydextrose, sucralose, and magnesium stearate. Packaged and irradiated under UV light for 30 minutes;
[0043] S2, passing D-mann...
Embodiment 3
[0046] Embodiment 3, a kind of direct compression type organic calcium vitamin D3 chewable tablet
[0047] The direct-compression organic calcium vitamin D3 chewable tablet consists of the following ingredients and parts by weight: 60 parts of calcium citrate, 50 parts of L-calcium lactate, 35 parts of microcrystalline cellulose, 5 parts of anhydrous glucose, and D-mannose 20 parts of sugar alcohol, 5 parts of polyethylene glycol 6000, 15 parts of vitamin D3, 0.9 parts of polydextrose, 5 parts of sucralose, 1.3 parts of magnesium stearate.
[0048] Such as figure 1 Shown, the preparation method of described direct compression type organic calcium vitamin D3 chewable tablet, comprises the steps:
[0049] S1, remove: calcium citrate, L-calcium lactate, microcrystalline cellulose, anhydrous glucose, D-mannitol, polyethylene glycol 6000, vitamin D3, polydextrose, sucralose, magnesium stearate Outer packaging, irradiated under ultraviolet light for 30 minutes;
[0050] S2, passi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com