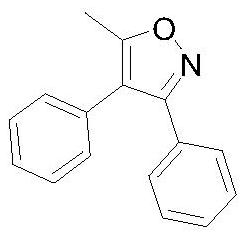
Synthetic method of 5-methyl-3, 4-diphenyl isoxazole
A technology of diphenylisoxazole and a synthesis method, applied in the field of medicine, can solve problems such as difficulty in obtaining raw materials, and achieve the effects of increased reaction continuity and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
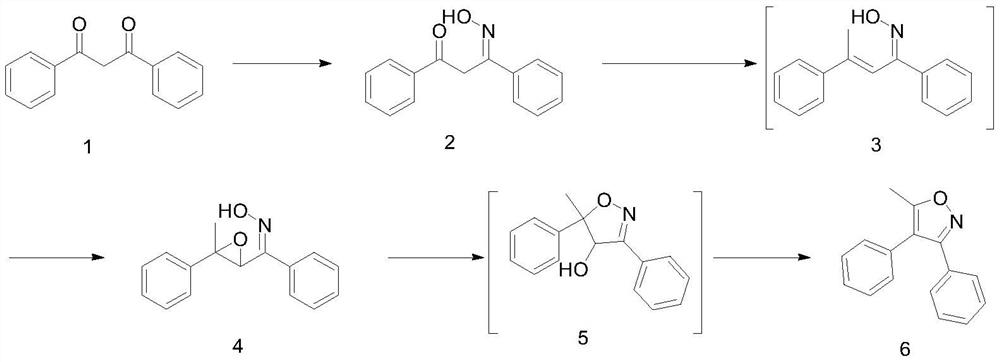
[0027] The first step: Synthesis of 1,3-diphenylpropane-1,3-diketone monoxime
[0028]
[0029] Under the protection of nitrogen, 112g (0.5mol, 1eq) of raw material I was added to 1000mL of 25% methanol aqueous solution, and the temperature was controlled at 25-32°C. 68.8g (0.495mol, 0.99) of hydroxylammonium hydrochloride was added in batches, and sodium acetate was added dropwise. The aqueous solution adjusts Ph=5.7. After the addition, react at 25°C for 24 hours, let it stand still, take the supernatant raw material ≤ 2%, concentrate under reduced pressure at 35-45°C, distill off methanol, cool down to 20-25°C, and filter , the filter cake was rinsed with water, and dried in vacuum at 35-45°C to obtain 112 g of 1,3-diphenylpropane-1,3-diketone monoxime, with a chemical purity of 96.1% and a yield of 93.6% as determined by HPLC. 1 HNMR (400MHz, DMSO-d6): δ=7.86(m,2H), 7.67-7.45(m,3H), 7.37-7.30(m,5H), 6.43(s,1H), 3.98(m,2H).
[0030]
[0031] Under nitrogen protection...
Embodiment 2
[0033] The second step: the synthesis of (3-methyl-3-phenyl-oxirane)-phenyl ketone oxime.
[0034]
[0035] Under the protection of nitrogen, dissolve 112g of intermediate II in 500g of tetrahydrofuran, cool down to -15°C, add dropwise (2M / L) methylmagnesium chloride tetrahydrofuran solution 665.2mL, after the dropwise addition, slowly raise the temperature to 5°C, and react for 1 hour. Add saturated ammonium chloride aqueous solution to quench, let stand to separate layers, extract the organic phase with 500mL of dichloromethane, combine the organic phases, add 102g of trifluoroacetic acid, heat up to 45°C and react for 5h, then sample LC intermediate 4≥87% , concentrated under reduced pressure to the remaining 1 volume, cooled to 10-25 ° C, added 300 g of n-heptane to make a slurry, filtered, and dried to obtain the intermediate (3-methyl-3-phenyl-oxirane)-phenylmethanol Ketoxime 100.4g, HPLC detection chemical purity 97.3%. Yield 85.2%, 1 HNMR (400MHz, DMSO-d6): δ=7.60...
Embodiment 3
[0039] The third step: the synthesis of 5-methyl-3,4-diphenylisoxazole.
[0040]
[0041] Under the protection of nitrogen, 4100g (0.395mol, 1eq) of the intermediate was dissolved in 700mL of dichloromethane, cooled to 0°C, and 511.9g (1.659mol, 4.2eq) of 46% boron trifluoride etherate was added dropwise, slowly Raise the temperature to 20°C and react for 2h. Sampling LC detection of raw materials <1%, the reaction solution cooled to 0-10 ° C, the reaction solution was added to 1N hydrochloric acid 500mL water quenched, static layering, the aqueous phase was extracted with 500mL of dichloromethane, the organic phase was combined, the organic phase Concentrate under reduced pressure to the remaining 3 volumes, then add 109 g of trifluoroacetic acid, raise the temperature to 40-45 ° C for 6 hours, take a sample for LC detection of intermediate 5 ≤ 1%, concentrate under reduced pressure to the remaining 2 volumes, add n-heptane to precipitate, filter get crude. The crude pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com