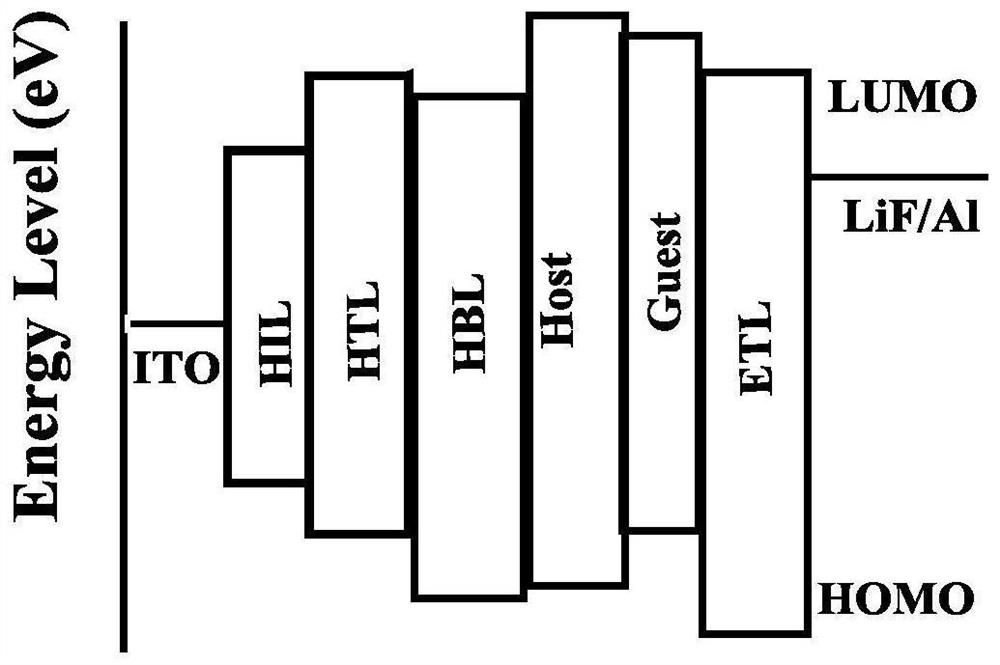
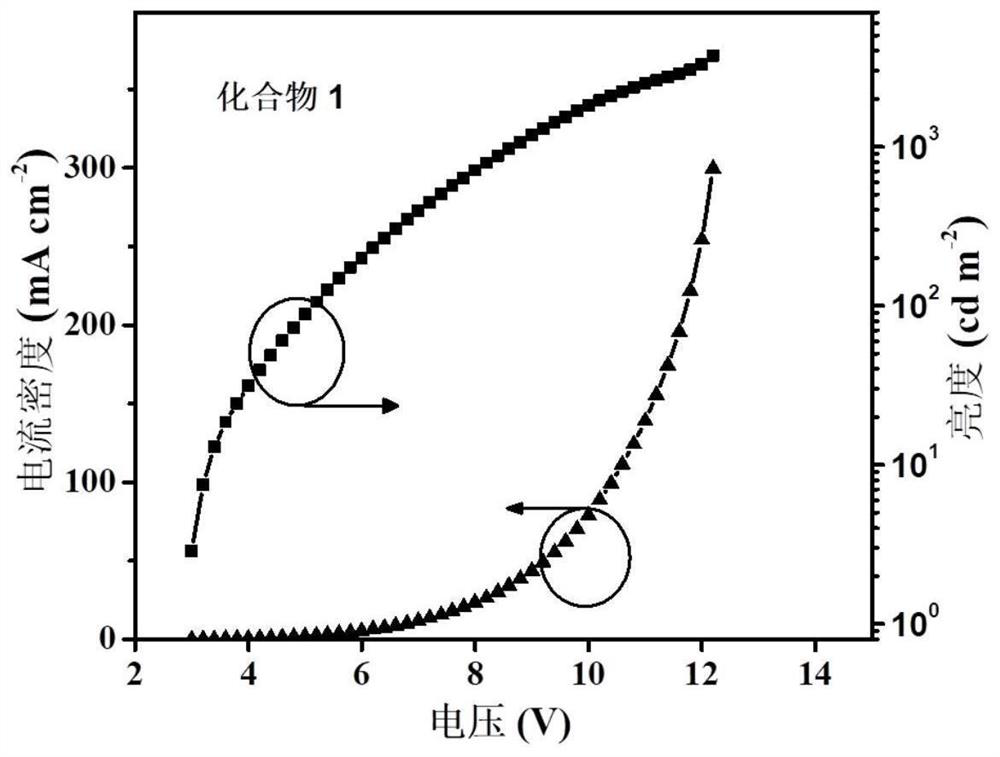
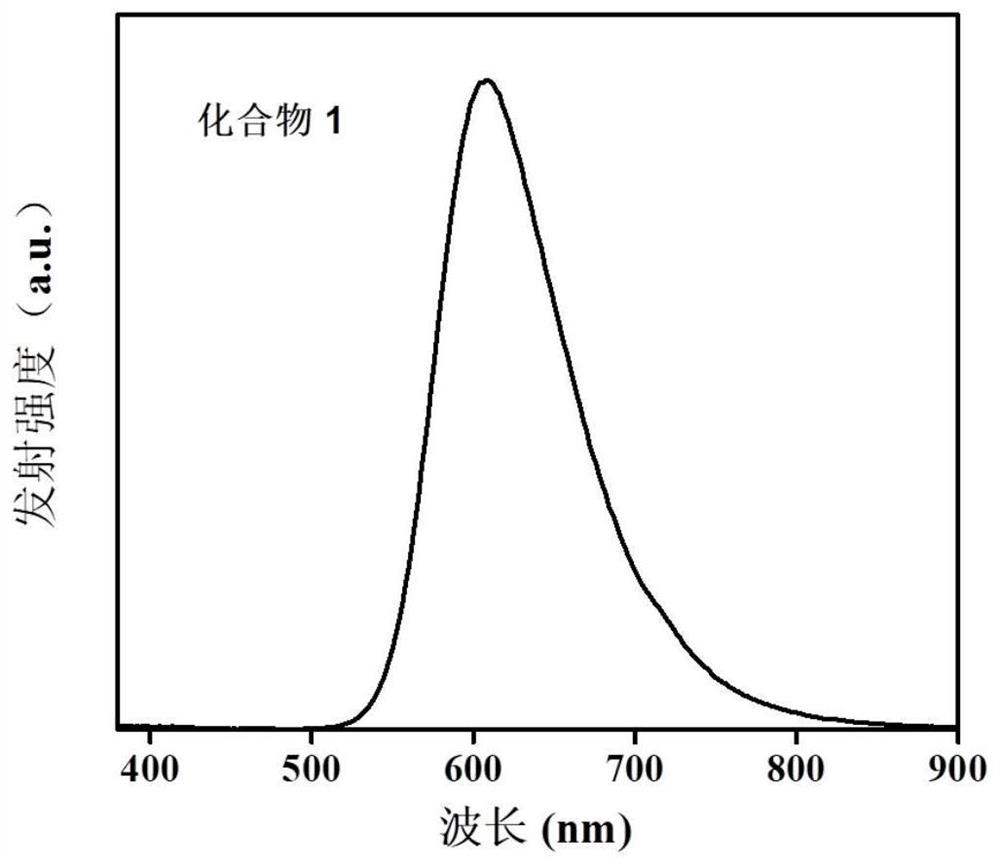
Naphthalimide azacyclo-luminescent material and application thereof
A luminescent material, naphthalimide technology, applied in luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of low efficiency and poor stability of luminescent materials, achieve good thermal stability, improved luminescence performance, good The effect of the application foreground
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Naphthalimide azacyclic luminescent material 1(3,4-bis(4-(diphenylamino)phenyl)-7H benzo[de]benzo[4,5]imidazo[ 2,1-a]isoquinolin-7-one) can be synthesized by the following method.
[0053]
[0054] (1) In a dry 500ml two-necked flask, add 1,4,5,8-naphthalene tetracarboxylic anhydride (10.03g, 37.3mmol), then add 350ml of water and stir to dissolve, then add potassium hydroxide (10.51g, 186.4 mmol), the solution was heated to 85°C, liquid bromine (4.7ml, 93.2mmol) was slowly added dropwise, heated and stirred for 1 hour, and after the reaction solution was cooled to room temperature, 20ml of hydrochloric acid was slowly added. After a large amount of solid was precipitated, it was suction-filtered, and the crude product was washed alternately with water and methanol three times to obtain intermediate 1-1 (4,5-dibromo-1,8-naphthalene dicarboxylic anhydride), with a yield of 91%.
[0055] (2) Add intermediate 1-1 (1.77g, 5mmol), phenylenediamine (0.54g, 5mmol), and 100...
Embodiment 2
[0058] The same preparation method as in Example 1, the difference is that the phenylenediamine in the reaction step (2) is replaced by 4',5'-diamino-[1,1':2',1'-triphenyl] -4,4'-dicarbonitrile, and finally the target product 3(4,4'-(3,4-bis(4-(diphenylamino)phenyl)-7-oxo-7H-benzo[de ]benzo[4,5]imidazo[2,1-a]isoquinoline-10,11-diyl)dibenzonitrile), the yield was 59%. MS(MALDI-TOF):m / z[M(H) + ] 960.076. Elemental analysis results (%): C 85.01; H 4.29; N 8.85; O 1.56.
Embodiment 3
[0060] The naphthalimide azacyclic luminescent material 13(10,11-bis(4-(diphenylamino)phenyl)-7H benzo[de]benzo[4,5]imidazo[ 2,1-a]isoquinolin-7-one) can be synthesized by the following method.
[0061]
[0062] (4) Add 4,5-dibromobenzene-1,2-diamine (1.33g, 5mmol), 1,8-naphthalene dicarboxylic anhydride (0.99g, 5mmol), and 100ml acetic acid solution in a dry 250ml single-necked flask , heated to reflux and stirred for 8 hours. After the reaction is complete, the intermediate 3-1(10,11-dibromo-7H-benzo[de]benzo[4,5]imidazo[2,1-a]isoquinoline-7- ketone), yield 80%.
[0063] (5) Intermediate 3-1 (1.27g, 3mmol), triphenylamine 4-boronate (2.43g, 8.4mmol), tetrakistriphenylphosphine palladium (0.34g, 0.3mmol), potassium carbonate (3.31g, 24mmol ), 12ml of water, 18ml of toluene, and 5ml of ethanol were sequentially added into a 100ml two-necked flask, stirred rapidly and passed a large amount of nitrogen for 10-15 minutes, and heated to reflux under nitrogen protection for 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com