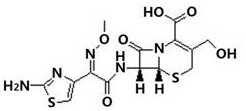
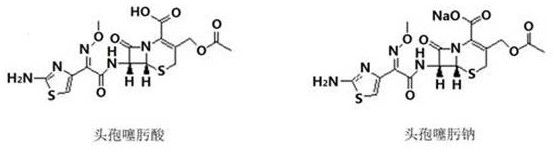
A kind of preparation method of 3-hydroxymethyl cefotaxime
A technology of hydroxymethyl cefotaxime and cefotaxime, which is applied in the fields of resisting vector-borne diseases, organic chemistry, fermentation, etc., can solve the problems of many steps and complicated operation, and achieve mild reaction conditions, simple and easy-to-control operation, The effect of high product conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: In a 500ml four-necked bottle, add 20g of cefotaxime acid and 160ml of purified water, control the temperature to 25°C to 30°C, stir evenly, add dropwise 3mol / L ammonia solution to adjust the pH to 7.0 to 7.5, and stir for 5min. Add 4g of cephalosporin C deacetylase, continue to use 3mol / L ammonia solution to fine-tune the pH to keep at 7.0-7.5, when the pH value remains unchanged for 5 minutes, the reaction ends, and then filter to remove the cephalosporin C deacetylase, collect filtrate;
[0025] When adjusting the above-mentioned filtrate temperature to 10~15 ℃, add dropwise the mixed solution of 5ml 36% concentrated hydrochloric acid and 7.5ml isopropanol, adjust the pH value to 2.5, crystallize, grow the crystal for 45min and filter, then wash with 30ml purified water. , dried at a drying temperature of 55 ° C and -0.090 MPa for 5 to 10 hours until the moisture content was less than 2.0% to obtain the target product of 3-hydroxymethyl cefotaxime as a pal...
Embodiment 2
[0030] Example 2: In a 500ml four-necked bottle, add 20g of cefotaxime acid and 200ml of purified water, control the temperature to 25°C to 30°C, stir evenly, add 3mol / L ammonia solution dropwise to adjust the pH to 7.0 to 7.5, and stir for 5min Then add 6g of cephalosporin C deacetylase, continue to use 3mol / L ammonia solution to fine-tune the pH to keep at 7.0-7.5, when the pH value remains unchanged for 5 minutes, the reaction ends, and then filter to remove the cephalosporin C deacetylase , collect the filtrate;
[0031]When adjusting the temperature of the above-mentioned filtrate to 10~15 ℃, dropwise add the mixed solution of 6ml 36% concentrated hydrochloric acid and 6ml ethanol, adjust the pH value to 2.0, crystallize, filter after cultivating the crystal for 45min, then wash with 20ml purified water, Vacuum drying at a drying temperature of 50 ° C and -0.095 MPa for 5 to 10 hours until the moisture content is less than 2.0% to obtain the target product of 3-hydroxymet...
Embodiment 3
[0032] Example 3: In a 500ml four-necked bottle, add 20g of cefotaxime sodium and 240ml of purified water, control the temperature to 25°C to 30°C, stir evenly, add 3mol / L ammonia solution dropwise to adjust the pH to 7.0 to 7.5, and stir for 5min After adding 8g of cephalosporin C deacetylase, continue to use 3mol / L ammonia solution to fine-tune the pH to keep at 7.0-7.5, when the pH value remains unchanged for 5 minutes, the reaction ends, and then filter to remove the cephalosporin C deacetylase , collect the filtrate;
[0033] When the temperature of the filtrate was adjusted to 10-15°C, a mixed solution consisting of 4ml of 85% phosphoric acid and 8ml of methanol was added dropwise, and the pH value was adjusted to 4.0 for crystallization. Vacuum drying at a temperature of 60 ° C and -0.085 MPa for 5 to 10 hours until the moisture content is less than 2.0% to obtain the target product of 3-hydroxymethyl cefotaxime as a pale yellow powder. The mass yield of the target prod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com