Method for detecting content of hydrazine substance in steroid hormone substance
A technology for steroid hormones and substance content, which is applied in measuring devices, instruments, scientific instruments and other directions to achieve the effects of high sensitivity, good selectivity and good accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A method for detecting the content of hydrazines in steroid hormones, comprising the steps of,
[0035] 1. The chromatographic column is Agela promosil C18 250*4.6mm, 5μm particle size or similar chromatographic column.
[0036] 2. The detection wavelength is 305nm, which is the wavelength at which the hydrazine hydrate derivative (benzaldehyde azine) has greater absorption in the ultraviolet-visible region.
[0037] 3. The preferred mobile phase is 0.3g / L edetate sodium solution-acetonitrile (30:70).
[0038] 4. The analysis time is 20 minutes.
[0039] 5. The content of benzaldehyde azine was calculated by the external standard method; the injection volume was 40ul; the flow rate was 1.0ml / min; the column temperature was room temperature.
[0040] 6. Benzaldehyde azine reference substance solution: Accurately weigh 25 mg of hydrazine sulfate reference substance, put it in a 100mL measuring bottle, add diluent to dissolve and dilute to the mark, shake well, accuratel...
experiment example
[0046] 1. Sample recovery data
[0047]
[0048] 2. Selective
[0049] By adding an equal amount of the reference substance solution to the system suitability mixture, and comparing the peak area of the hydrazine sulfate derivative with the reference substance solution, it is concluded that there is no influence on the detection of hydrazine hydrate under the condition that other impurities exist.
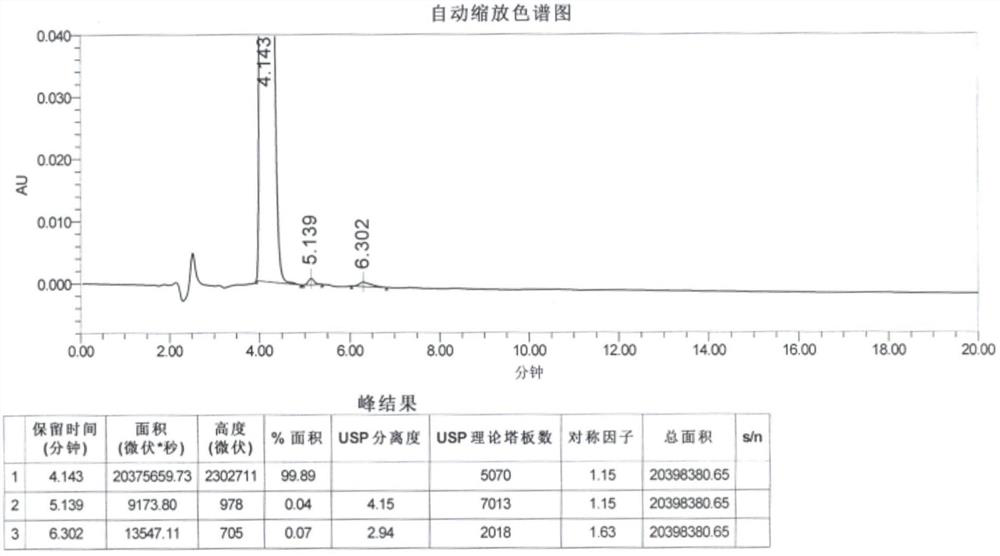
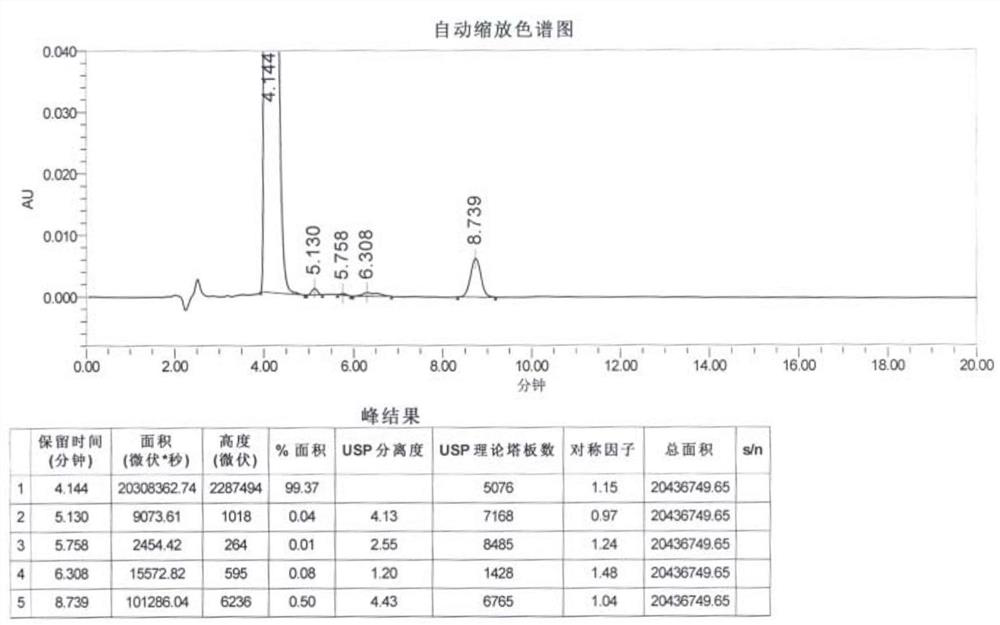
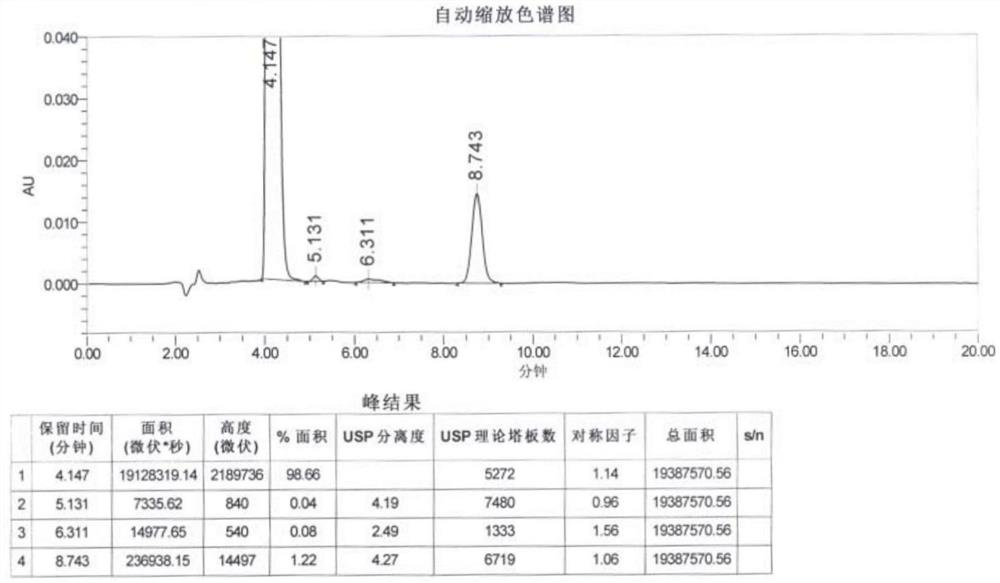
[0050] The peak area of hydrazine sulfate derivative in the reference substance solution ( Figure 4 ): 11508.55;
[0051] The peak area of hydrazine sulfate derivative in the system suitability solution ( Figure 5 ): 11255.81.
[0052] 3. Sensitivity
[0053] The limit of quantification of the method was calculated to be about 0.03ppm by the signal-to-noise ratio of the limit of quantification not less than 10:1. Has a high sensitivity.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com