A kind of multi-response nitrogen-heterocyclic formaldehyde fluorescent probe molecule and its preparation method and application
A fluorescent probe and nitrogen heterocycle technology is applied in the field of multi-responsive nitrogen heterocycle formaldehyde fluorescent probe molecules and their preparation, which can solve the problems of complex probe molecular structure, many preparation steps, slow response speed and the like, and achieve molecular structure The effect of stability, many recognition sites, and short response time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: the preparation of probe molecule Ia
[0036]
[0037] (1) In a 100 ml round bottom flask, two (2-pyridyl) ketones (1mmol), methyl 4-formylbenzoate (1mmol) and ammonium acetate (10mmol) were dissolved in 30 ml of glacial acetic acid, Reflux reaction under rapid stirring for 9 hours; after the completion of the reaction, cool to room temperature, pour the reaction solution into ice water with stirring, adjust the pH=7 with ammonia water, filter the obtained solid under reduced pressure, wash with water 3 times, and wash the crude product with ethanol - recrystallization from acetone mixed solvent, and vacuum drying to obtain a yellow solid, which is intermediate IVa.
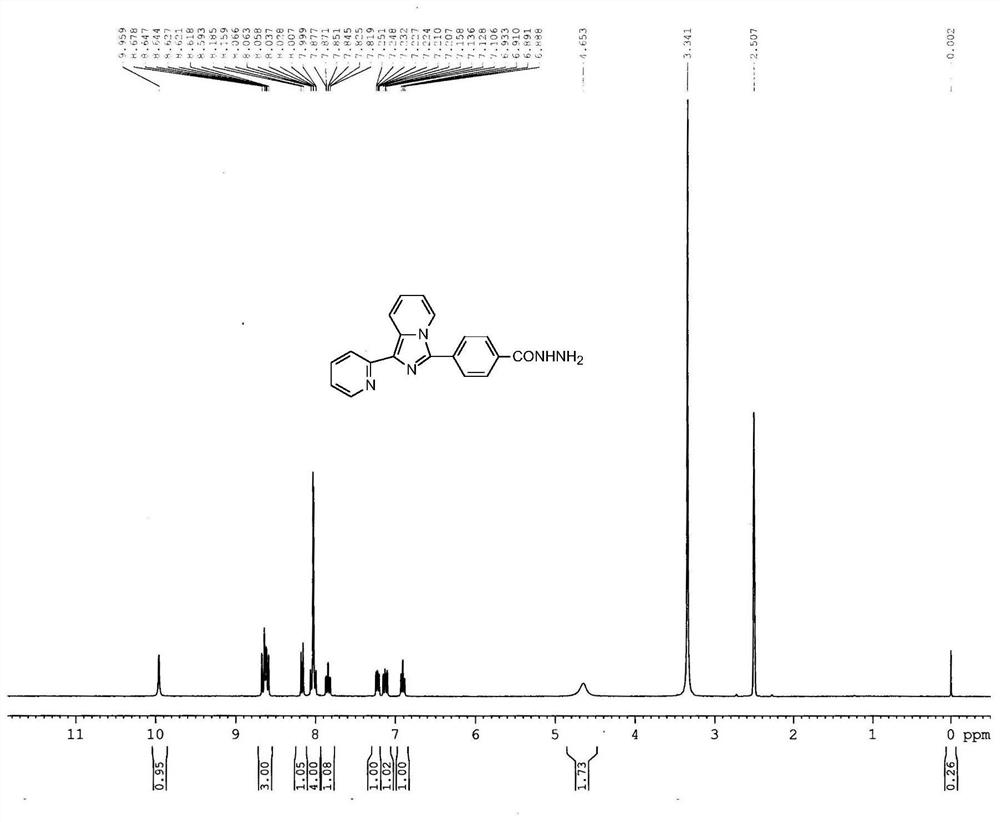
[0038] 1 H NMR (300MHz, CDCl 3 / TMS)δ:3.97(s,3H),6.71(t,J=7.5Hz,1H),6.93-6.99(m,1H),7.09-7.14(m,1H),7.70-7.76(m,1H) ,7.94-7.98(m,2H),8.15-8.31(m,4H),8.65(m,1H),8.75(d,J=7.5,Hz,1H); 13 C NMR (75MHz, CDCl 3 / TMS) δ: 52.28, 114.54, 120.04, 120.73, 121.51, 121.55, 122.06, 127.83, 129.94, ...
Embodiment 2
[0042] Preparation of probe molecule Ia
[0043] (1) In a 100 ml round bottom flask, dissolve bis(2-pyridyl)methanone (1mmol), methyl 4-formylbenzoate (1.2mmol) and ammonium acetate (17mmol) in 30 ml of glacial acetic acid , reflux reaction under rapid stirring for 7 hours; after the completion of the reaction, cool to room temperature, pour the reaction solution into ice water under stirring, adjust the pH=7 with 10wt% sodium hydroxide or potassium hydroxide aqueous solution, and filter the obtained solid under reduced pressure , washed with water for 3 times, the crude product was recrystallized from ethanol-acetone mixed solvent, and dried in vacuo to obtain intermediate IVa.
[0044] (2) In a 100 milliliter round bottom flask, intermediate IVa and hydrazine hydrate (mass concentration is 90wt%) were dissolved in 50 milliliters of ethanol at a weight ratio of 1:9, and refluxed for 7 hours under rapid stirring, and the reaction was completed After cooling to room temperatur...
Embodiment 3
[0046] UV-Vis Absorption and Fluorescence Properties Test of Probe Molecules
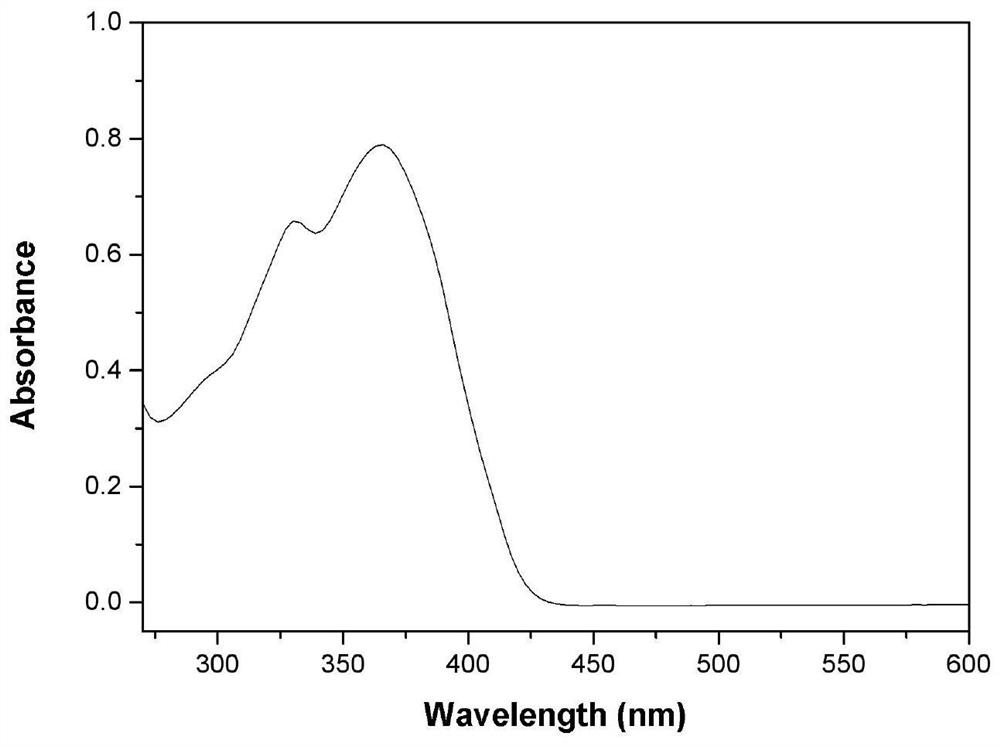
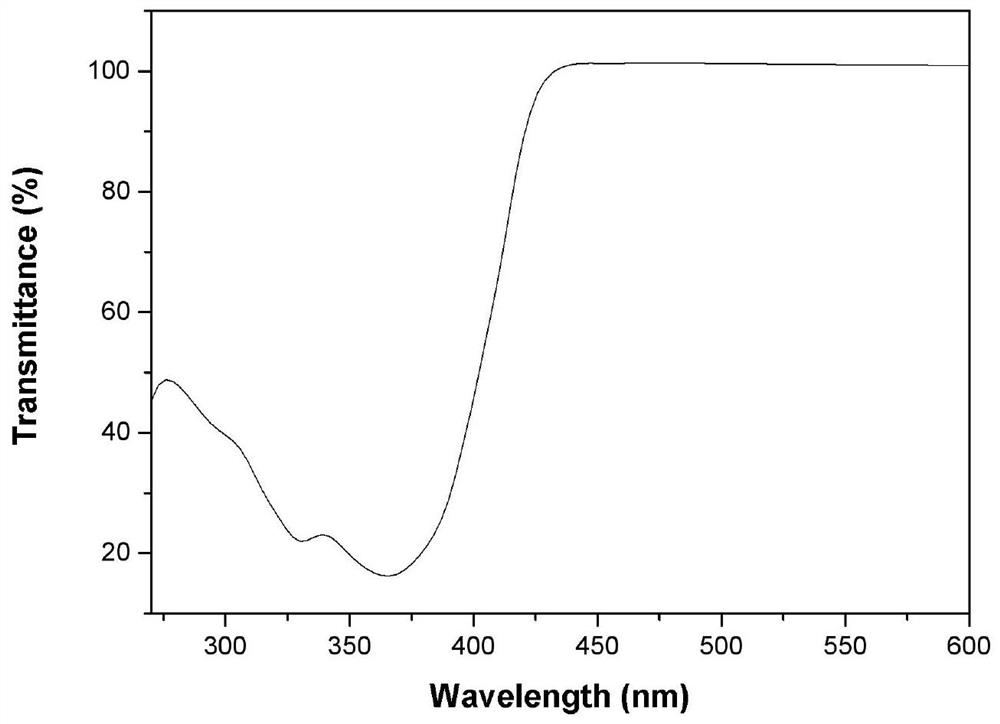
[0047] Compound Ia was formulated to a concentration of 1 × 10 -5 The N,N-dimethylformamide solution of M, measured the ultraviolet absorption and fluorescence properties on the HORIBA JobinYvonAqualog absorption and three-dimensional fluorescence scanning spectrometer with a 1 cm sample cell, the results are as follows Figure 2-4 shown.
[0048] Depend on figure 2 It can be seen that compound Ia has broadband strong absorption characteristics in the 270nm-430nm band, presents a strong absorption band, and has two absorption peaks, respectively located at 330nm and 366nm, the absorption wavelength of the maximum absorption peak is 366nm, and at greater than 430nm There is no obvious absorption in the above bands. Depend on image 3 It can be seen that in the spectral region greater than 430nm, the transmittance of the molecules is greater than 98%, and has good transparency. The results show ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com