Guanidyl organic compound and preparation method and application thereof
An organic compound and guanidine-based technology, applied in the field of guanidine-based organic compounds and their preparation, can solve problems such as reducing the antibacterial effect, and achieve the effects of reducing toxicity, strong antibacterial property and high electrochemical impedance value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] In a specific example, the preparation method includes the following steps: respectively dissolving the above-mentioned first compound and cyanamide in an acid solution, then mixing and reacting at 5-15° C. for 48-72 hours, and the reaction chemical formula is as follows.
[0053]
[0054] In a specific example, the molar ratio of the first compound to cyanamide is (1-1.2): 1, and the first compound of the raw material is slightly excessive, so that the conversion rate of the synthesis reaction is maximized and the cyanamide (cyanamide) is reacted as completely as possible .
[0055] Optionally, the acid solution is a common strong acid solution such as hydrochloric acid solution, sulfuric acid solution, nitric acid solution and the like. Preferably, the pH of the acid solution is 4-5, and by controlling the pH, side reaction hydrolysis and self-polymerization of cyanamide can be avoided. Preferably, when dissolved, the molar ratio of the first compound to the acid ...
Embodiment 1
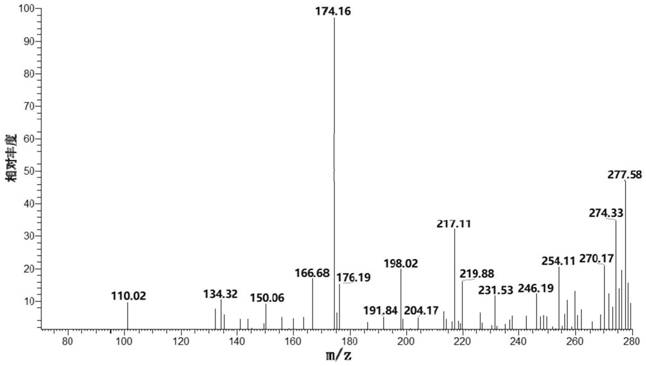
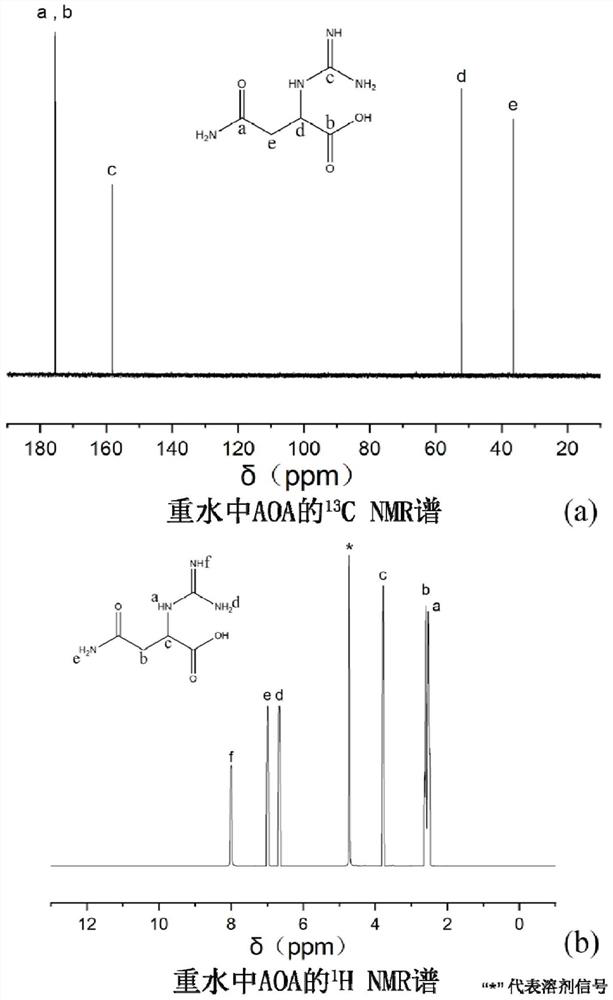
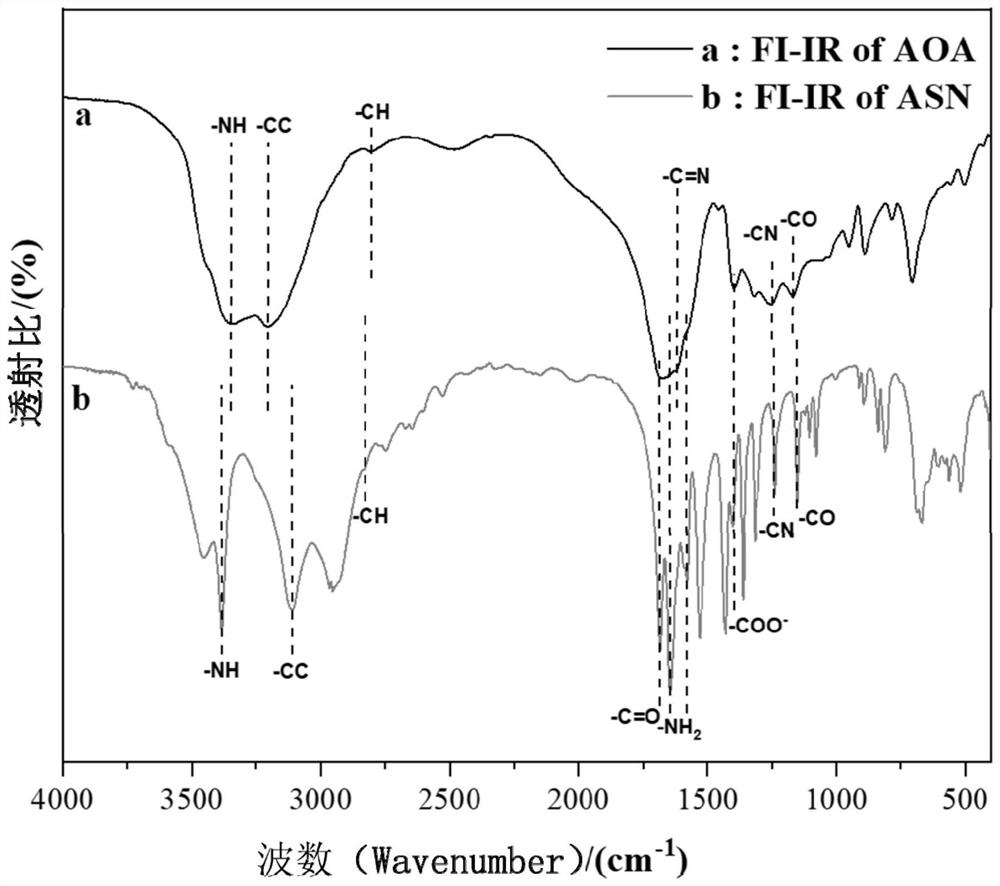
[0091] Synthesis of 4-amino-2-((hydrazinomethylimine)amino)-4-oxobutanoic acid, a guanidine-based organic compound
[0092] Weigh 33 g of asparagine and dissolve it in 0.5 M hydrochloric acid solution with a volume of 1 L. Weigh 10.5 g of cyanamide and dissolve it in 1 L of 0.5 M hydrochloric acid solution. After mixing and stirring the above reaction solution evenly, the mixture was uniformly and continuously stirred and sealed for reaction at 5° C. under the condition of pH=4-5, and reacted for 72 hours.
[0093] The reaction product was rotary evaporated until the reaction solvent basically disappeared, filtered, and the precipitate was washed to complete the primary crystallization, and the crystals were harvested. The crystals were washed with a large amount of deionized water, and then re-dissolved in deionized water, followed by rotary evaporation, recrystallization, and filtration. The recrystallized crystals were washed with a large amount of deionized water again an...
Embodiment 2
[0098] Take lithium nitrate 8g, lithium carbonate 1g, lithium hydroxide 1g, sodium fluoride 2g, sodium nitrite 1g, simethicone 1g, the obtained 4-amino-2-((hydrazinomethyl sulfide) of embodiment 1 Amine)amino)-4-oxobutanoic acid 2.8g, aspartic acid 1g, 1H,1H,2H,2H-perfluorooctyltriethoxysilane 1.4g, 1H,1H,2H,2H-perfluoro 1.4 g of decyltrimethoxysilane was dissolved in 1000 mL of deionized water, and the pH of the above solution was adjusted to 10 with nitric acid to prepare a surface treatment solution for a lithium-aluminum hydrotalcite conversion film (see Table 2 for names of different hydrotalcite ratios).
[0099] Table 2 The treatment liquid formula table of the lithium aluminum hydrotalcite conversion film of embodiment 2
[0100]
[0101] To prepare the 6N01 aluminum alloy test piece, firstly use 400, 800, 1200# sandpaper to manually polish the aluminum alloy surface, then immerse the aluminum alloy test piece in the self-made alkaline lotion formula for 5 minutes, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Water contact angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com