Inonotus obliquus alcohol F and application thereof in preparation of alpha-glucosidase inhibitor drugs
A technology of Inonotus obliquus alcohol and glucosidase, applied in the preparation of α-glucosidase inhibitor drugs, in the field of triterpenoids, can solve problems such as toxic side effects and heart system risks, and achieve postprandial control blood sugar effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
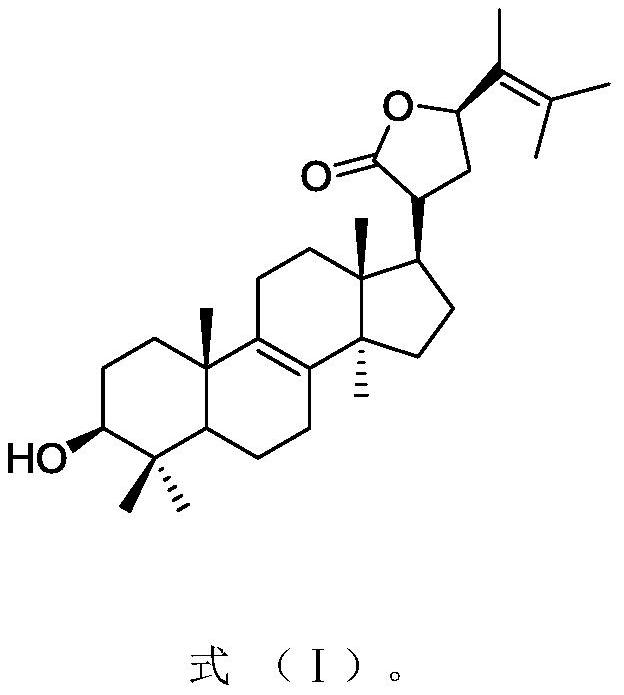
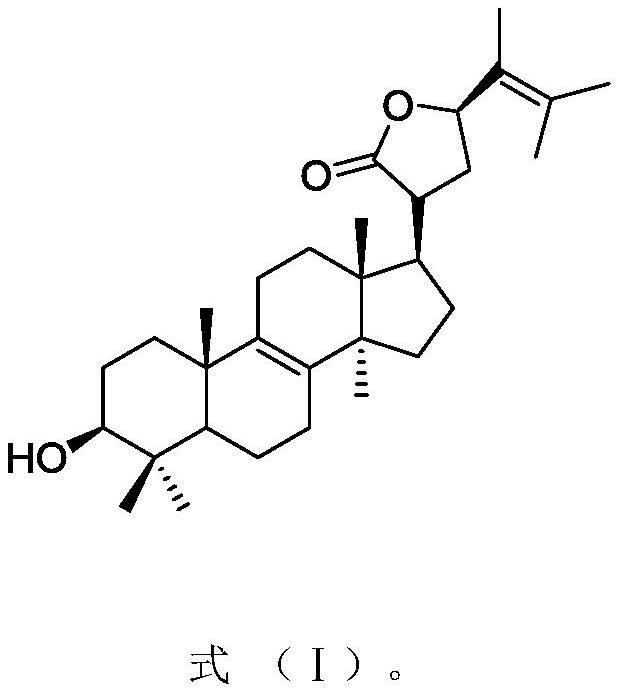
[0026] Embodiment 1: the preparation of inonotofaciol F
[0027] Take 5 kg of dried sclerotia of Polyporaceae fungus Ganoderma lucidum (Inonotus obliquus), crush it, and heat (80°C) reflux extraction with 10 times the amount (mass / volume, g / mL) of 95% ethanol for 2 times. After 2 hours, the extracts were combined and concentrated under reduced pressure to 1 / 20 of the original volume to obtain IO; 3 times the amount of ethyl acetate was added to extract twice to obtain the ethyl acetate extract IO-EA. IO-EA was dissolved with 2 times the amount (mass / volume, g / mL) of chloroform methanol (1:1 v / v) solution, and evenly mixed into the silica gel. After the solvent was evaporated, the adsorbed ethyl acetate extract IO-EA Silica gel is added to the top of the adsorbent in the chromatographic column for silica gel column chromatography separation, with chloroform-methanol at a volume ratio of 100:0, 98:2, 95:5, 90:10, 80:20, 70:30 , 50:50 and 0:100 as the eluent gradient elution, co...
Embodiment 2
[0028] Embodiment 2: the preparation of inonotofaciol F
[0029] Inonotus obliquus mycelium was inoculated on PDB medium, and cultured at 25°C with 200r / min shaking for 7 days to prepare seed liquid, and then inoculated into 20 Erlenmeyer flasks containing rice medium according to 5% inoculation amount, and cultured statically at 25°C On 45d, the fermented product was obtained. The rice culture medium is composed of the following components: 70g / bottle of rice, 100mL / bottle of purified water, which is prepared by adding 70g of rice into a bottle filled with 100ml of water and sterilizing. The fermented product was added to ethyl acetate for soaking and ultrasonic extraction for 3 times, and the extract was concentrated to dryness under reduced pressure to obtain a crude extract. The crude extract was passed through a silica gel column, eluted with cyclohexane and methanol, and the fraction eluted with methanol was collected and concentrated to dryness. Then carry out medium ...
Embodiment 3
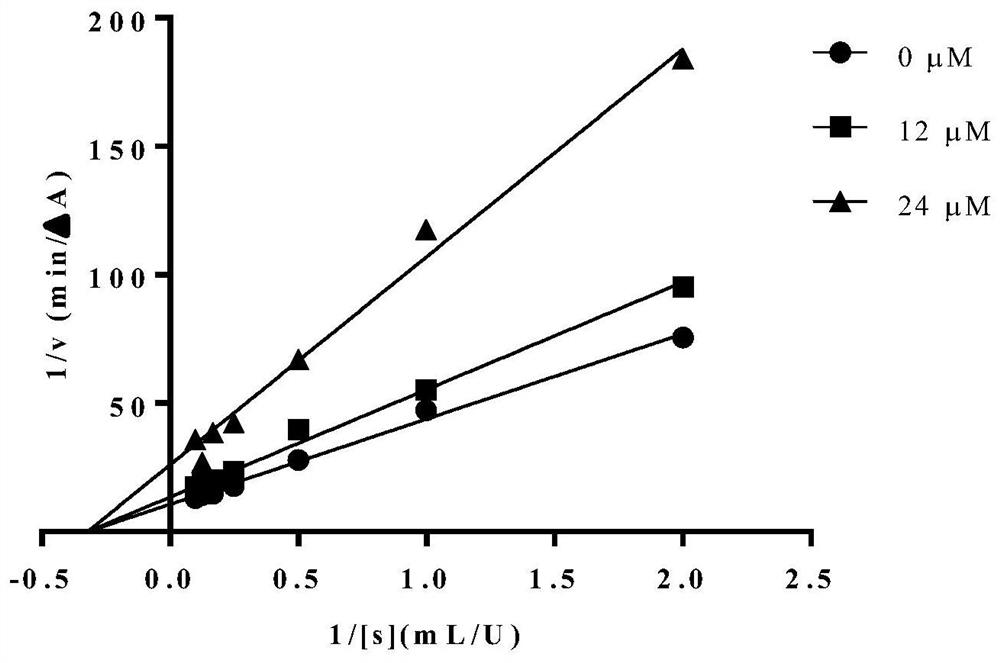
[0036] Example 3: In vitro inhibition of α-glucosidase activity test of Inonotolidinol F
[0037] Prepare a series of test sample solutions with different concentrations in advance (dissolve in a small amount of DMSO, dilute to the corresponding concentration with distilled water, control the final volume fraction of DMSO2 CO 3 (1M) Terminate the reaction, and measure the absorbance at a wavelength of 405 nm. A blank group was set up in the experiment at the same time: use 10 μL of phosphate buffer (50 mM, pH7.0) instead of the test sample solution; blank control group: use 10 μL of phosphate buffer (50 mM, pH7.0) instead of the test sample solution, At the same time, 50 μL of phosphate buffer (50 mM, pH 7.0) was used to replace the aqueous solution of α-glucosidase; sample control group: 50 μL of phosphate buffer (50 mM, pH 7.0) was used to replace the aqueous solution of α-glucosidase. Each of the above experimental groups was repeated three times, and the results were aver...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com