Degradable medical elastomer material and application thereof
A technology of elastomer material and composite material, used in stent for pancreatic duct drainage, degradable elastomer material and its application in the fields of ureter, bile duct, and biomedical materials, which can solve the limitation, the degradation speed of the elastomer is fast, and the drainage time cannot be reached. and other problems to achieve the effect of ensuring no breakage, reliable fixing effect and stable size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
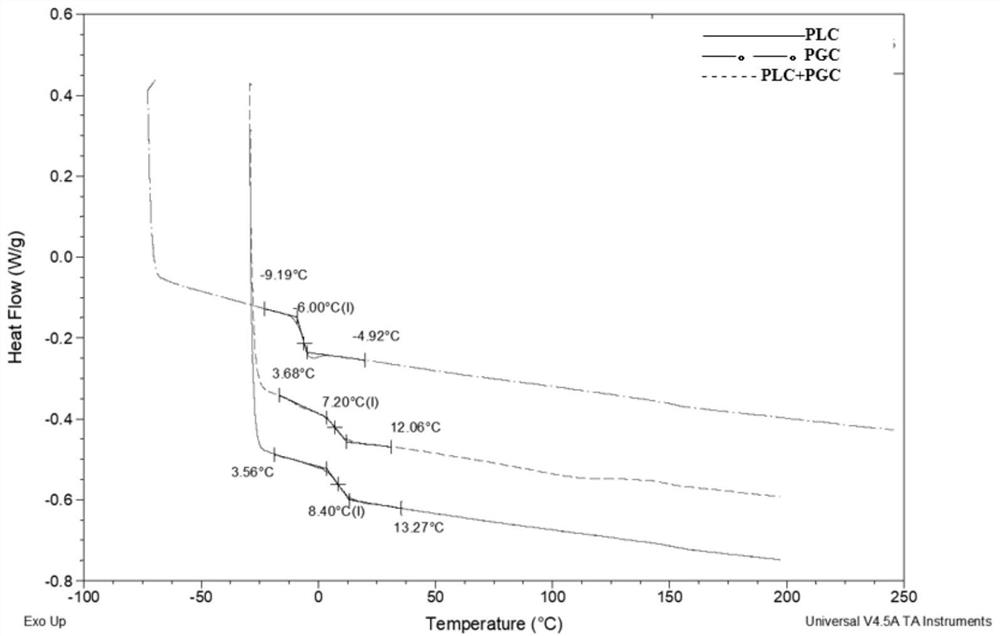
[0044] The preparation of embodiment 1 L-lactide / ε-caprolactone copolymer
[0045] 540 grams of recrystallization and purification of L-lactide (LLA) and 500 grams of ε-caprolactone monomer (CL) of vacuum dehydration and purification are placed in a 5000ml reactor, and then 0.015% stannous octoate catalyst is added , Polymerized at 150°C for 24 hours under the protection of nitrogen to obtain L-lactide / ε-caprolactone copolymer. The copolymer was dissolved in dichloromethane, precipitated with ethanol, and the precipitate was dried in a vacuum dryer at 60° C. for 48 hours to obtain L-lactide / ε-caprolactone copolymer 1 (PLC1). The product is made into a chloroform solution with a concentration of 0.1% by weight, and its intrinsic viscosity is tested at 30° C. with an Ubbelohde viscometer; 1 The ratio of L-lactide and ε-caprolactone segments is determined by H spectrum; the above materials are made into dumbbell strips with a thickness of 1mm or 2mm on a flat vulcanizing machine...
Embodiment 2
[0050] The preparation of embodiment 2 glycolide / ε-caprolactone copolymer
[0051] The glycolide monomer (GA) of 540 grams of recrystallization purification treatment and the 480 grams of epsilon-caprolactone monomer (CL) of vacuum dehydration purification treatment are placed in 3000ml reactor, add the stannous octoate catalyst of 0.02% again , under the protection of nitrogen, raise the temperature of the system to 150°C, react for 1 hour, then raise the temperature to 180°C and react for 12 hours, transfer the polymer out of the reactor, further crush it into particles smaller than 3mm, put it into 90 ℃ in a vacuum oven for 50 hours to remove unreacted monomers to obtain glycolide / ε-caprolactone copolymer 1 (PGC1). The product was made into a 0.1% by weight hexafluoroisopropanol solution, and its intrinsic viscosity was tested at 30°C with a Ubbelohde viscometer; with hexafluoroisopropanol as a solvent, the 1 The ratio of glycolide and ε-caprolactone segments is determined...
Embodiment 3
[0056] Example 3 Preparation of L-lactide / ε-caprolactone copolymer and glycolide / ε-caprolactone copolymer composite (PLC / PGC)
[0057] Dissolve 300 grams of the above-mentioned L-lactide / ε-caprolactone copolymer 1 (PLC1) and 100 grams of glycolide / ε-caprolactone copolymer (PGC1) in methylene chloride solution, stir and mix evenly , and then mixed the blended polymer solution with ethanol as a precipitating agent, washed the precipitated polymer, and vacuum-dried at 60° C. for 48 hours to obtain the PLC / PGC composite material 1 of the present invention. The above materials are made into dumbbell strips at 140°C-170°C on a flat vulcanizing machine by means of thermocompression molding. According to the method of Example 1, the tensile strength, elongation at break, Shore hardness A value and strength maintenance time of the composite material were tested. The preparation of the PLC / PGC composite material can also be realized by the following method.
[0058] The L-lactide / ε-ca...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Outer diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com