Antibody against alpha-hemolysin and application thereof
An antibody, VH-CDR1 technology, applied in the direction of antibodies, applications, antibody mimics/stents, etc., can solve the problems of limited antibiotics, clinical patient treatment failure and high mortality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Example 1 Recombinant Expression of Staphylococcus aureus α-Hemolysin (α-Toxin) Fused with His Tag
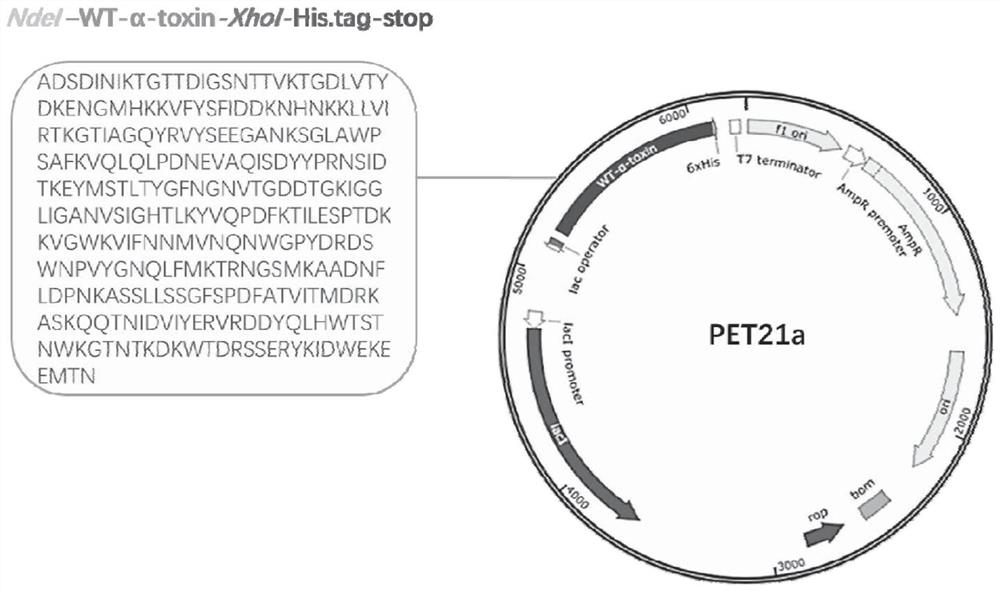
[0094] Taking the amino acid sequence of Staphylococcus aureus α-hemolysin as the target sequence, the corresponding base sequence was artificially synthesized, and cloned into the Pet-21a plasmid containing His tag by using restriction sites NdeI and XhoI. Wherein the Staphylococcus aureus α-hemolysin amino acid sequence is shown in SEQ ID NO: 78, the corresponding base sequence is shown in SEQ ID NO: 79, and the construction of the recombinant plasmid is shown in figure 1 .
[0095] The obtained recombinant plasmid was transformed into competent cells BL21(DE3)pLysS, and a single colony was picked and inoculated into LB liquid medium containing 100 μg / ml ampicillin the next day, and cultured overnight at 37°C with shaking. Inoculate the overnight cultured bacterial solution into LB liquid medium containing 100 μg / ml ampicillin at a volume ratio of 1:100, shake at 20...
Embodiment 2
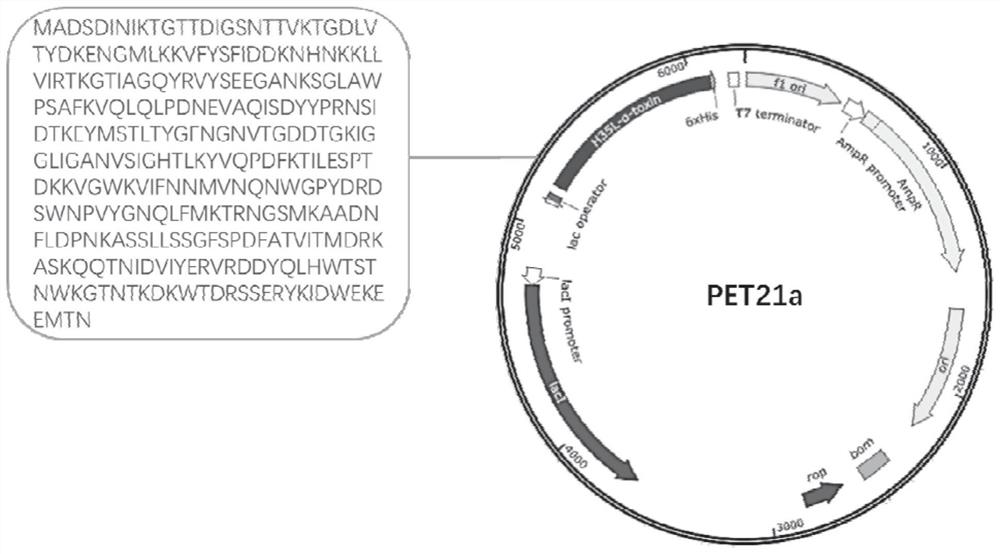
[0096] Example 2 Recombinant Expression of Staphylococcus aureus α-Hemolysin Mutant (H35Lα-Toxin) Fused with His Tag
[0097] Based on the amino acid sequence of Staphylococcus aureus α-hemolysin, the 35-position histidine (His) was mutated into leucine (Leu) to obtain the mutated amino acid sequence, and the corresponding base sequence was artificially synthesized, and the enzyme cleavage site was used to Click NdeI and XhoI to clone it into the Pet-21a plasmid containing His tag. Wherein the Staphylococcus aureus mutant α-hemolysin (H35Lα-Toxin) amino acid sequence is shown in SEQ ID NO: 80, the corresponding base sequence is shown in SEQ ID NO: 81, and the construction of the recombinant plasmid is shown in figure 2 .
[0098] The obtained recombinant plasmid was transformed into competent cells BL21(DE3)pLysS, and a single colony was picked and inoculated into LB liquid medium containing 100 μg / ml ampicillin the next day, and cultured overnight at 37°C with shaking. I...
Embodiment 3
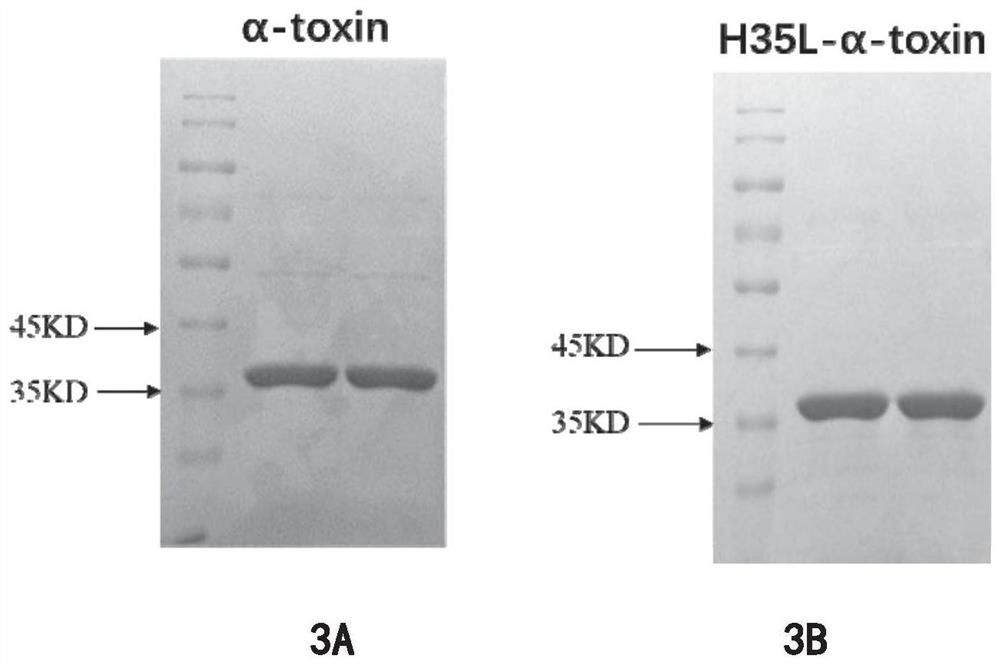
[0099] Example 3 Purification of Staphylococcus aureus α-hemolysin (α-Toxin) and its mutant (H35Lα-toxin)
[0100] The Escherichia coli induced to express Staphylococcus aureus α-hemolysin and its mutants were disrupted with a sonicator, and worked at 180W for 3s and 3s, with a time of 7-9min; centrifuged at 13,000rpm for 30min, collected the supernatant, and passed through a 0.22μmL filter Filter sterilize.
[0101] The Ni column and the filtered supernatant were mixed on a rotary mixer for 1 h at room temperature, and the Ni column was loaded into the packed column. Use 5 times the column bed volume of BD solution (containing imidazole concentration 30mM) to elute the protein non-specifically bound to the Ni column, wash until the protein chromogenic solution does not change color, and then use 5 times column bed volume BB solution (containing imidazole concentration 300mM ) to elute the target protein. Then use a 10KD concentrator tube to concentrate and replace the elu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com