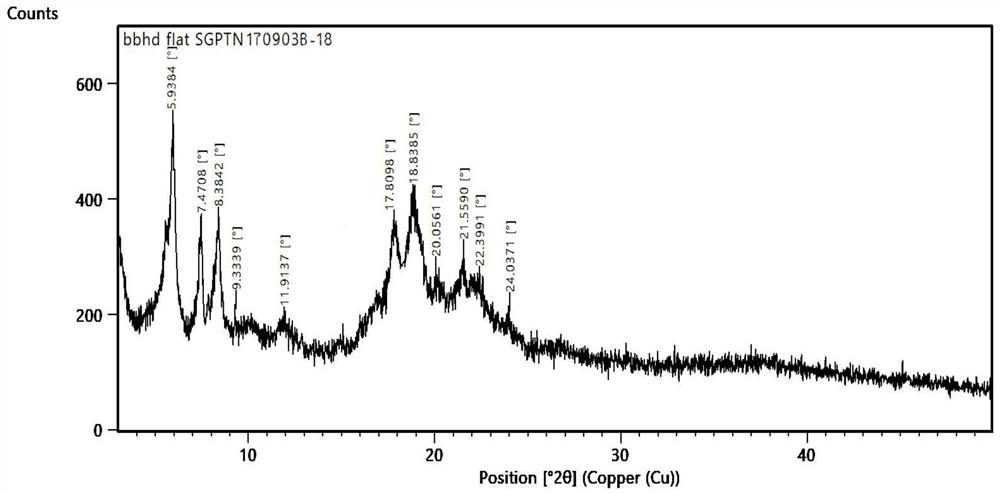
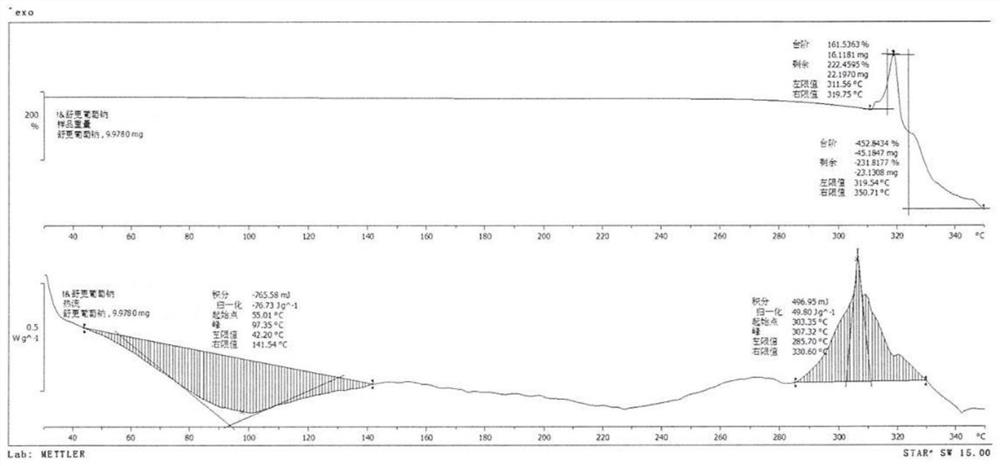
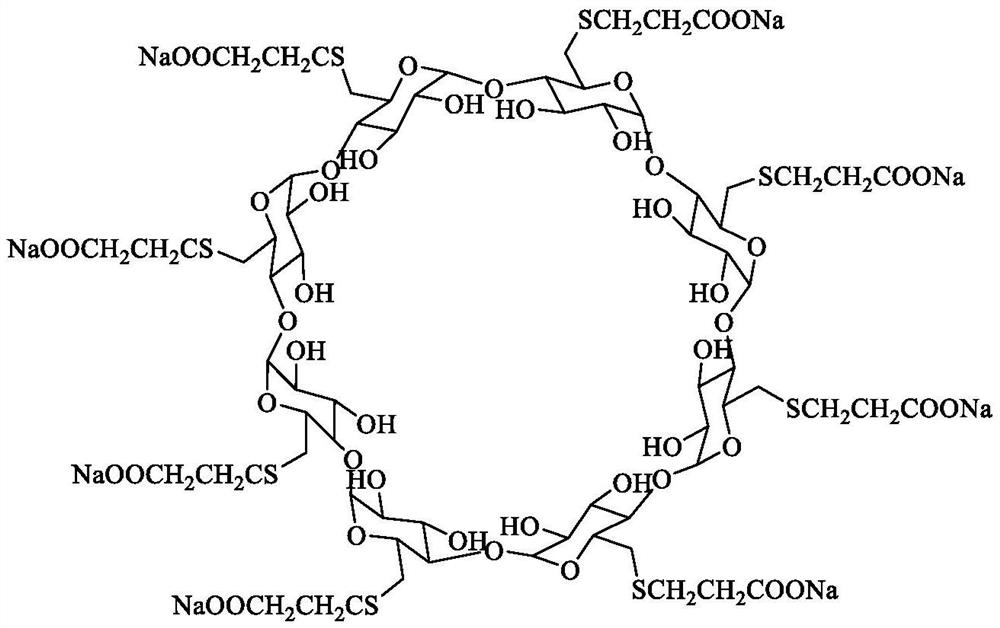
Sugammadex sodium crystal form M
A technology of sugammadex sodium and crystal form, applied in the field of crystal form drug molecules, can solve the problems of thermal stability, photostability, dissolution, and bioavailability that cannot be well satisfied, and is suitable for large-scale promotion. Simple application, preparation method, and color-qualified effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Under the protection of argon, add sugammadex sodium (10.03g) into purified water (15ml), put it into an ultrasonic instrument (45KHz) and stir to dissolve; add methanol (20ml) to the sugammadex sodium aqueous solution, and control the temperature Ultrasonic reaction at 35°C for 2 hours; when the reaction is complete, the temperature of the reaction liquid is controlled by ultrasonic (45KHz) at 25°C, and acetone (150ml) is added dropwise while stirring. , and the filter cake was dried under reduced pressure at 40°C to constant weight to obtain white crystalline sugammadex sodium with a yield of 98.8% and a purity of 99.91%.
Embodiment 2
[0054] Under the protection of argon, add sugammadex sodium (10.06g) into purified water (10ml), put it into an ultrasonic instrument (50KHz) and stir to dissolve; add ethanol (10ml) to the sugammadex sodium aqueous solution, and control the temperature Ultrasonic reaction at 30°C for 3 hours; after the reaction, the temperature of the reaction solution was controlled by ultrasonic (50KHz) at 25°C, and methanol (100ml) was added dropwise while stirring. , and the filter cake was dried under reduced pressure at 40°C to constant weight to obtain white crystalline sugammadex sodium with a yield of 97.4% and a purity of 99.85%.
Embodiment 3
[0056] Under the protection of argon, add sugammadex sodium (10.04g) into purified water (20ml), put it into an ultrasonic instrument (45KHz) and stir to dissolve; add methanol (25ml) to the sugammadex sodium aqueous solution, and control the temperature Ultrasonic reaction at 40°C for 2 hours; after the reaction, the temperature of the reaction solution was controlled at 20°C under ultrasonic (45KHz), and acetonitrile (200ml) was added dropwise while stirring. , and the filter cake was dried under reduced pressure at 40°C to constant weight to obtain white crystalline sugammadex sodium with a yield of 97.6% and a purity of 99.82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com